Sonidegib, a hedgehog pathway inhibitor, is indicated for treatment of locally advanced basal cell carcinoma, based on the results of the BOLT study. However, to date, no real-world study of sonidegib has been reported. An observational, retrospective, single-centre study (PaSoS study) was conducted. The primary objective was to evaluate the efficacy of sonidegib for treatment of locally advanced basal cell carcinoma in a real-world setting. Secondary objectives included modalities of use, tolerability, tumour evolution, and management after discontinuation. A total of 21 patients treated with sonidegib were included from March 2018 to January 2021. The median follow-up was 18.7 months and median exposure 7.0 months. Objective response (OR) rate was 81.0% (n = 17) including 6 (29%) patients with a complete response (CR). Disease control rate was 100%. First tumour response was rapid, with a median time of 2.3 months. Nine (43%) patients underwent surgery after sonidegib discontinuation, and no relapse was observed. All the patients experienced at least 1 adverse event (AE). Muscle spasms were the most frequent AE (n = 14; 67%), followed by dysgeusia (n = 8; 38%) and alopecia (n = 12; 57%). The efficacy and safety profile of sonidegib in this first-to-date real-life trial are consistent with prior results. Overall, real-world evidence corroborated sonidegib efficacy and tolerability as a first-line treatment for locally advanced basal cell carcinoma.
Key words: sonidegib; basal cell carcinoma; real-world evidence.
Accepted May 23, 2022: Epub ahead of print May 23, 2022
Acta Derm Venereol 2022; 102: adv00740.
DOI: 10.2340/actadv.v102.1995
Corr: Florian Herms, AP-HP Department of Dermatology, Université de Paris, DMU ICARE, INSERM U976 HIPI, Team 1 Hôpital Saint-Louis, FR-75010 Paris, France. E-mail: florian.herms@aphp.fr
SIGNIFICANCE
Sonidegib, a hedgehog pathway inhibitor, is indicated for treatment of locally advanced basal cell carcinoma. This observational, retrospective, single-centre study was the first-to-date trial to evaluate efficacy and safety of sonidegib in locally advanced basal cell carcinoma in clinical practice. A total of 21 patients treated with sonidegib were included. Overall, all patients benefited from therapy, either with a response or stabilization of disease. Eighty-one percent of patients responded to treatment, of which 29% achieved a complete response. Response was rapid. The efficacy and safety profile of sonidegib in this first-to-date real-life trial are consistent with prior results. In conclusion, real-world evidence corroborated sonidegib efficacy and tolerability as a first-line treatment for locally advanced basal cell carcinoma.
INTRODUCTION
Skin malignancies are a major public health concern, and their prevalence is steadily increasing as the population ages (1). Basal cell carcinoma (BCC) is the most common type of skin cancer, especially in Caucasians (2); it accounts for approximately 80% of the non-melanoma skin cancers (NMSC).
Most localized BCCs are managed by surgical removal, topical treatment, or photodynamic therapy. However, in locally advanced basal cell carcinoma (laBCC) or in rare metastatic BCC (mBCC) standard treatment options are not accessible (3). Targeted treatment with a Hedgehog pathway inhibitor (HHI) has become the first treatment strategy in such cases (4, 5). BCC tumours feature genetic alterations in the Hedgehog (Hh) signalling pathway, resulting in aberrant pathway activation and uncontrolled cellular proliferation. The Hh signalling pathway is a key regulator of cell differentiation, proliferation, and organogenesis during embryonic development (6–8). Mutations in the human homologs of Drosophila patched (PTCH1), smoothened (SMO), and suppressor of fused (SUFU), resulting in an abnormal hedgehog signalling pathway, have been found in more than 95% of sporadic BCCs. Gorlin syndrome, also known as nevoid basal cell carcinoma syndrome, is an autosomal dominant neurocutaneous disease characterized mostly by a PTCH1 germline mutation (9, 10).
Sonidegib, a selective antagonist of the SMO receptor, was approved by the European Medicines Agency (EMA) in August 2015, for patients with laBCC who are not amenable to curative surgery or radiation therapy (11). Approval of sonidegib 200 mg in laBCC was based on the results of the BOLT study (12). A 30-month analysis of updated long-term efficacy and safety data supported the long-term efficacy of sonidegib in patients with laBCC (13). For the approved dosage of 200 mg sonidegib, objective response (OR) rates were 60.6% (central review) and 74.2% (investigator review) per BCC-RECIST (Response evaluation criteria in solid tumors)-like criteria; median duration of response (DOR) was 26.1 months (15.7 months per investigator review); median duration of progression-free survival (PFS) were 22.1 months (central review) and 19.4 months (investigator review) (13). These results were confirmed in the final 42-month analyses of the BOLT study, the longest follow-up with a HHI to date (14, 15).
To date, only case reports and cases series have been published about the use of sonidegib in the real-word setting. The aim of the current retrospective study was to collect more evidence on the efficacy and safety of sonidegib administered under real-life conditions in patients presenting laBCC at a French cancer centre.
PATIENTS AND METHODS
Patients
A retrospective cohort study that included all patients ≥ 18 years with histologically proven BCC not amenable to radiation therapy, curative surgery, or other local therapies that received sonidegib at 200 mg daily, according to label, between March 2018 and January 2021 was analysed. Patients were included regardless of the number of courses of treatment prior to the initiation of sonidegib. Approximately 20 patients with laBCC were expected to be documented at 1 site.
Study design
A single-centre, observational, non-comparative retrospective study (PaSoS study) was conducted at the oncodermatology department of the Saint-Louis Hospital to collect data on effectiveness, safety, and utilization of sonidegib in patients with laBCC in routine clinical practice. According to the article R1121-1 of the French Public Health Code, being a retrospective study, the study was not subject to ethics committee’s favourable opinion and competent authority authorization.
Data collection
Patients still alive at the time of data collection signed a consent form. In the case of a deceased patient who did not refuse the use of their personal data for scientific research (as indicated in their medical files) beforehand, data were collected. Charts of all patients who did not oppose data collection were reviewed. The medical files for patients included in the study were analysed up to January 2021.
Study aims and evaluation criteria
The primary objective of this study was to evaluate the effectiveness of sonidegib for the treatment of laBCC in real-world settings in terms of OR rate (sum of complete response (CR) rate and partial response (PR) rate, as assessed by the principal investigator (PI)). The secondary endpoints of this study were: stable response rate (not fulfilling the criteria for PR, CR, or progression), disease control rate (DCR) (sum of CR, PR, and stabilization rates), DOR (time from first observed objective response (CR or PR) until disease progression or death due to any cause), PFS (time from treatment initiation until disease progression or death), time to first tumour response, time to maximal response, recurrence after surgery, overall survival (OS), modalities of use of sonidegib, occurrence of adverse events (AE), tumour evolution after sonidegib discontinuation, modalities of management of laBCC after discontinuation of sonidegib.
Statistical analysis
Statistical analysis was performed with the statistical package SAS (version 9.4 or later). An analysis plan detailing the analyses performed was prepared by the study statistician and approved by the PI). All patients included were analysed.
Primary endpoint analysis. The OR rate was estimated with a 2-sided 95% confidence interval (95% CI) (Clopper-Pearson method).
Secondary endpoint analyses. Proportions were estimated with a 2-sided 95% CI (Clopper-Pearson method). Time-to-event-based analyses were carried using the Kaplan–Meier method. Median time and 2-sided 95% CIs were computed.
Safety analysis. The occurrence of AEs was tabulated by their frequency, broken down by their system-organ class and severity. Patients who had at least 1 occurrence of a specific AE were described as a number and a percentage.
RESULTS
Patient characteristics
A total of 21 patients were treated at the centre for laBCC using sonidegib between March 2018 and January 2021. The median follow-up was 18.7 months. Baseline clinical and demographic characteristics are shown in Table I. Patients were between 44.0 and 91.0 years of age, with a median of 75.0 years. Thirteen (62%) patients were male. The weight at sonidegib initiation ranged from 53.0 to 90.0 kg (median 68.5 kg). Among those, Eastern Cooperative Oncology Group score (ECOG) status was available for 16 patients. The vast majority of patients (n = 11; 69 %) were totally functional (ECOG=0). The remainder (n = 5; 31%) had an ECOG score of 1. For 5 patients, data were unavailable. The median age of patients at the time of laBCC diagnosis was 71.4 years. The median duration from laBCC diagnosis and the start of sonidegib treatment was 3.7 months. Twenty patients (95%) had laBCCs located on the head, and 1 (4%) patient was diagnosed with a Gorlin-Goltz syndrome. Table II summarizes the patients’ laBCC history. At baseline, 13 laBCCs (62%) had an aggressive histological subtype. Before sonidegib initiation, 8 patients (38%) had undergone surgical excision, with a median number of 2 surgeries reported. Previous pharmacological treatments were documented in 3 (14%) patients. All of them received vismodegib, interrupted for intolerable AE. Patients had 1 target lesion. The mean maximum diameter of the target lesion was 4.1 ± 3.2 cm (median 3.3 cm). Most patients (86%) reported at least 1 relevant comorbidity. Most frequent comorbidities included cardio-vascular disorders (n = 7; 33%). No patient was immunosuppressed at the time of sonidegib initiation.

Treatment exposure
Sonidegib was initiated at 200 mg daily in all patients. The median duration of sonidegib exposure from initiation to permanent discontinuation or end of follow-up was 7.0 months (95% CI 4.2–12.4 months). Out of the 21 patients, 18 (86%) permanently discontinued sonidegib. Main reasons documented at the time of permanent discontinuation were AEs (n = 8; 44%) and planned surgery (n = 6; 33%). Temporary discontinuation occurred in 4 (19%) patients because of AEs.
Efficacy data
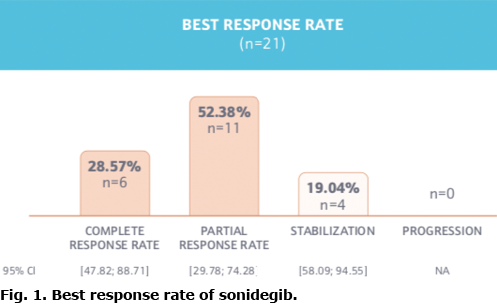
Primary endpoint: objective response rate. During sonidegib treatment, 81% of patients achieved an OR (n = 17; 95% CI 59–95%), of which 6 (29%) patients achieved a CR, 11 (52%) demonstrated a PR at best, and the remainder (n = 4; 19%) were stable with no tumour progression. The best overall response is shown in Fig. 1. The first observation of the OR was primarily documented through clinical/photography evaluation (n = 16; 76%). Imaging evaluations were performed on 5 (24%) of the patients, whereas histology was performed on 1 (4%) of the patients.
Secondary endpoints. Disease control was observed in all the patients (100%; 95% CI 84–100%) during sonidegib treatment. The median time to first tumour response was 2.3 months (95 % CI 1.1–3.3 months). The median time to maximal tumour response was 3.2 months (95% CI 2.2–3.9 months). The duration of OR was assessed in the 16 patients who had a recorded response and a subsequent visit that allowed for a second assessment. One patient had a response at the last, most recent visit. Therefore, this data was not included in the follow-up analysis. The mean DOR was 14.1 ± 1.3 months. PFS was calculated from treatment initiation of sonidegib until the first reported disease progression, death, or final information. However, the median PFS could not be estimated (mean=16.0 ± 1.1 months).
Finally, no death occurred during the study recollection period.
Secondary analysis after sonidegib discontinuation. Nine (43%) patients underwent surgery after sonidegib discontinuation (including 6 previously described planned surgery). Cryotherapy (n = 1) and radiation (n = 1) were administered after the use of sonidegib.
Out of the 9 patients who had surgery, none experienced disease progression.
The tumour evolution from the discontinuation of sonidegib to the last follow-up visit was assessed in patients who did not have surgery (n = 9). Six of them improved or maintained their response, but 3 relapsed.
Safety data
Change in sonidegib posology. Change in sonidegib prescription occurred in 19 (91%) patients during the recollection period. Two (10%) had their frequency of administration reduced from daily to every 2 days (as indicated in label to help manage AEs), 4 (19%) had their treatment interrupted temporarily, and 18 (86%) had their sonidegib treatment permanently discontinued. The primary cause for modifying sonidegib prescription was AEs (n = 11; 58%).
Adverse events. The most common AE are summarized in Fig. 2. A total of 71 AEs were reported during sonidegib exposure for all patients included in the study. Muscle spasms were the most common AEs reported by treated patients (n = 14; 67%). Alopecia (n = 12; 57%), dysgeusia (n = 8; 38%), weight decrease (n = 5, 24%), asthenia (n = 4, 19%), blood creatinine phosphokinase increased (n = 3, 14%), ageusia (n = 3, 14%), and decreased appetite (n = 3, 14%) were also reported.
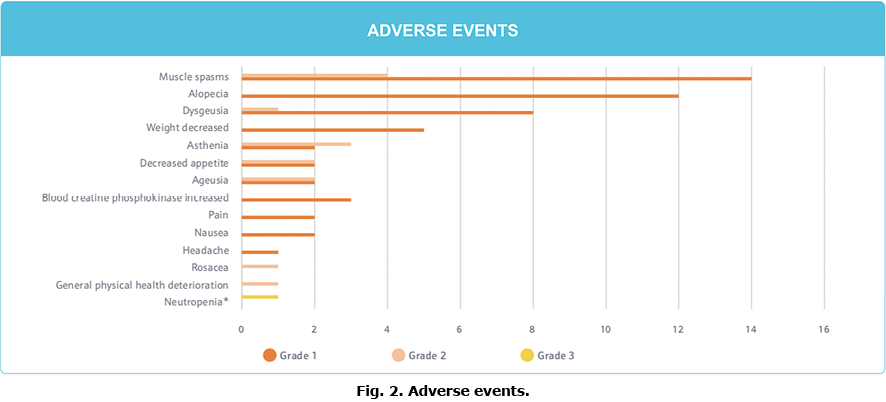
Except for 1 episode of neutropaenia, all of the AEs were grade 1 or 2. This neutropaenia was thought to be related to the treatment and led to the temporary suspension of sonidegib. The incident resolved without sequelae, and there was no resurgence upon resumption of sonidegib.
DISCUSSION
The advent of HHIs (vismodegib and sonidegib) has transformed the therapeutic paradigm for advanced BCC, resulting in substantial clinical improvement for these patients. The pivotal trials ERIVANCE and BOLT, demonstrated an OR assessed through Investigator Review of 60.3% and 74.2% for laBCC, respectively (13, 16).
To date, several clinical cases have been published, demonstrating sonidegib’s rapid activity and great tolerance, but larger sample size clinical investigations are required to validate these findings in clinical practice (17–19). The current study is the first conducted in patients with laBCC treated with 200 mg in real life.
Despite the constraints of paralleling interventional and non-interventional clinical trials, the findings of the PaSoS study can be placed, with caution, in the perspective of the pivotal phase II BOLT trial. In this trial, the OR for patients treated with sonidegib was 81% (n = 17; 95% CI 60–95%) including 6 (29%) patients with a complete response (CR). These observations are consistent with earlier findings from the long-term 30-month analysis of the pivotal phase II BOLT study and recently published 42-month analysis with an OR of 74.2% per investigator review using RECIST-criteria and the reported OR of 76.8% in a retrospective case series conducted in 13 patients (14, 19).
In addition, the rate of disease control was excellent, as it was observed in all patients (100%; 95% CI 84–100%). The median time to first tumour responses was 2.3 [95% CI 1.1; 3.3] months, which was consistent with the prior duration reported after 12 months of follow-up in the BOLT trial (2.5 [95% CI 1.99–3.7] months per investigator review) (20). Notably and reported for the first time in this study, the time to maximal response was 3.2 [95 % CI 2.2; 3.9] months, indicating that the patient’s response peaked close to their first response.
Interestingly, approximately one-third of patients interrupted their treatment for planned surgery (33%). Although not explicitly stated as a rationale for effectiveness, this suggested that sonidegib therapy achieved significant tumour shrinkage to qualify for surgical intervention.
In this study, the median PFS (mean 16.0 ± 1.1 months) could not be calculated, and no deaths occurred throughout the follow-up period. In addition, there was no tumour progression in any of the 9 patients who had lesion resection.
Sonidegib demonstrated a manageable safety profile, similar to that described previously. All patients experienced at least one AE, all except one of which were mild. Physicians have a pivotal part to play providing the best possible AE management for patients: e.g. on-label dose reduction for sonidegib (11), dose interruptions (11, 21) and even supportive medications (22).
Only when patients develop secondary resistance under HHI therapy, they may avail the approved second-line treatment with cemiplimab (23), with a lower response rate of 30%. If there is a relapse after discontinuation of a HHI, re-challenge with HHI-therapy seems to be the most effective approach, with response rates of 85% (24, 25).
While the current study has a number of limitations, including small sample size, missing data due to its retrospective nature, variability in follow-up schedules, and follow-up duration, it was demonstrated that efficacy in this largest-to-date real-life study of sonidegib is consistent with previously reported results. The response to treatment with sonidegib is rapid, and the safety profile is similar to that previously described. Overall, real-world evidence corroborated the efficacy and tolerability profile of sonidegib as a first-line treatment for laBCC.
Conflicts of interest: FH and NBS declare consulting for Sun Pharma, outside the submitted work.
REFERENCES
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71: 209–249.
- Ridky TW. Nonmelanoma skin cancer. J Am Acad Dermatol 2007; 57: 484–501.
- Villani A, Fabbrocini G, Costa C, Scalvenzi M. Sonidegib: safety and efficacy in treatment of advanced basal cell carcinoma. Dermatol Ther (Heidelb) 2020; 10: 401–412.
- Peris K, Fargnoli MC, Garbe C, Kaufmann R, Bastholt L, Seguin NB, et al. Diagnosis and treatment of basal cell carcinoma: European consensus-based interdisciplinary guidelines. Eur J Cancer 2019; 118: 10–34.
- Work G, Invited R, Kim JYS, Kozlow JH, Mittal B, Moyer J, et al. Guidelines of care for the management of basal cell carcinoma. J Am Acad Dermatol 2018; 78: 540–559.
- Rubin AI, Chen EH, Ratner D. Basal-cell carcinoma. N Engl J Med 2005; 353: 2262–2269.
- Taipale J, Beachy PA. The Hedgehog and Wnt signalling pathways in cancer. Nature 2001; 411: 349–354.
- Scales SJ, de Sauvage FJ. Mechanisms of Hedgehog pathway activation in cancer and implications for therapy. Trends Pharmacol Sci 2009; 30: 303–312.
- McMillan R, Matsui W. Molecular pathways: the hedgehog signaling pathway in cancer. Clin Cancer Res 2012; 18: 4883–4888.
- Abe Y, Tanaka N. Roles of the hedgehog signaling pathway in epidermal and hair follicle development, homeostasis, and cancer. J Dev Biol 2017; 5: 12.
- Summary of product characteristics. https://www.ema.europa.eu/en/medicines/human/EPAR/odomzo#product-information-section.
- Migden MR, Guminski A, Gutzmer R, Dirix L, Lewis KD, Combemale P, et al. Treatment with two different doses of sonidegib in patients with locally advanced or metastatic basal cell carcinoma (BOLT): a multicentre, randomised, double-blind phase 2 trial. Lancet Oncol 2015; 16: 716–728.
- Lear JT, Migden MR, Lewis KD, Chang ALS, Guminski A, Gutzmer R, et al. Long-term efficacy and safety of sonidegib in patients with locally advanced and metastatic basal cell carcinoma: 30-month analysis of the randomized phase 2 BOLT study. J Eur Acad Dermatol Venereol 2018; 32: 372–381.
- Gutzmer R, Robert C, Loquai C, Schadendorf D, Squittieri N, Arntz R et al. Assessment of various efficacy outcomes using ERIVANCE-like criteria in patients with locally advanced basal cell carcinoma receiving sonidegib: results from a preplanned sensitivity analysis. BMC Cancer 2021; 21: 1244.
- Dummer R, Guminksi A, Gutzmer R, Lear JT, Lewis KD, Chang ALS, et al. Long-term efficacy and safety of sonidegib in patients with advanced basal cell carcinoma: 42-month analysis of the phase II randomized, double-blind BOLT study. Br J Dermatol 2020; 182: 1369–1378.
- Sekulic A, Migden MR, Basset-Seguin N, Garbe C, Gesierich A, Lao CD, et al. Long-term safety and efficacy of vismodegib in patients with advanced basal cell carcinoma: final update of the pivotal ERIVANCE BCC study. BMC Cancer 2017; 17: 332.
- Villani A, Fabbrocini G, Costa C, Scalvenzi M. Complete remission of an advanced basal cell carcinoma after only 3-month treatment with sonidegib: report of a case and drug management during COVID-19 pandemic. Dermatol Ther 2020; 33: e14200.
- Wang K, Patel M, Prabhu AV, Lewis GD. First reported case of concurrent sonidegib and radiotherapy for recurrent, advanced basal cell carcinoma. Rep Pract Oncol Radiother 2021; 26: 149–152.
- Villani A, Costa C, Fabbrocini G, Scalvenzi M. Systemic hedgehog inhibitors to treat locally advanced basal cell carcinomas of the head-neck region: a retrospective study. Dermatol Ther 2021: e15036.
- Dummer R, Guminski A, Gutzmer R, Dirix L, Lewis KD, Combemale P, et al. The 12-month analysis from Basal Cell Carcinoma Outcomes with LDE225 Treatment (BOLT): a phase II, randomized, double-blind study of sonidegib in patients with advanced basal cell carcinoma. J Am Acad Dermatol 2016; 75: 113–125.e5.
- Erivedge – Summary of product characteristics. https://www.ema.europa.eu/en/medicines/human/EPAR/erivedge#product-information-section.
- Lacouture ME, Dreno B, Ascierto PA, Dummer R, Basset-Seguin N, Fife K, et al. Characterization and management of hedgehog pathway inhibitor-related adverse events in patients with advanced basal cell carcinoma. Oncologist 2016; 21: 1218–1229.
- Romero D. Cemiplimab is a new option in BCC. Nat Rev Clin Oncol 2021; 18: 400.
- Herms F, Lambert J, Grob JJ, Haudebourg L, Bagot M, Dalac S, et al. Follow-up of patients with complete remission of locally advanced basal cell carcinoma after vismodegib discontinuation: a multicenter French study of 116 patients. J Clin Oncol 2019; 37: 3275–3282.
- Bassompierre A, Dalac S, Dreno B, Neidhardt EM, Maubec E, Capelle C et al. Efficacy of sonic hedgehog inhibitors rechallenge, after initial complete response in recurrent advanced basal cell carcinoma: a retrospective study from the CARADERM database. ESMO Open 2021; 6: 100284.