There is a need for biomarkers that predict the success of transplantation of venous leg ulcers (with autologous split-thickness skin grafts). The primary objective of this exploratory study was to investigate the association between split-thickness skin graft healing in venous leg ulcers and candidate wound fluid biomarkers representing inflammatory cell and endogenous proteinase activities, and bioactivity. A secondary objective was to compare biomarker levels of the 17 venous leg ulcers with sterile split-thickness skin graft donor-site wounds in another 10 patients with venous leg ulcers. Wound fluids were collected for 24 h using a validated method. The concentration of preoperative matrix metalloproteinase-9 in wound fluid was higher in venous leg ulcers showing good healing (n = 10) than in venous leg ulcers showing poor healing (n = 7) 12 weeks after transplantation with meshed split-thickness skin grafts. The diagnostic value of matrix metalloproteinase-9 was good according to receiver-operating characteristic curve analysis. Matrix metalloproteinase activity in wound fluids from split-thickness skin graft donor-site wounds increased as a function of time and healing, but was still lower than matrix metalloproteinase activity in venous leg ulcer wound fluids, which showed increased levels of most biomarkers except for matrix metalloproteinase-9 and matrix metalloproteinase-2. In conclusion, wound fluid matrix metalloproteinase-9 concentration is a potential predictive biomarker of split-thickness skin graft healing in venous leg ulcers.
Key words: venous leg ulcer; proteinase; cytokine; biomarker; wound healing; keratinocyte.
Accepted May 23, 2022; Epub ahead of print May 23, 2022
Acta Derm Venereol 2022; 102: adv00749.
DOI: 10.2340/actadv.v102.201
Corr: Klaus Kirketerp-Møller, Copenhagen Wound Healing Center and Department of Dermatology, Bispebjerg Hospital, University of Copenhagen, Copenhagen, Denmark. E-mail: kkm@dadlnet.dk
SIGNIFICANCE
Venous leg ulcers heal slowly, if at all. Although transplantation with an autologous skin graft is used to promote healing it is resource-demanding and accompanied by risks of postoperative complications. A bedside diagnostic tool would be extremely helpful to the clinician to decide whether a patient with venous leg ulcer is suitable for skin transplantation. This exploratory study identified matrix metalloproteinase-9 in wound fluid as a potential biochemical predictor of graft healing. This promising initial finding needs to be examined further in larger clinical trials.
INTRODUCTION
Chronic wounds are common, with a global prevalence of 1%. Most venous leg ulcers (VLUs) heal with optimal treatment, including compression of the lower extremity. However, a cohort consisting of 505 patients with VLUs had a mean time to healing of 3.0 months, while only 53% healed within 12 months (1). Risk factors for non-healing are deep venous insufficiency and a history of deep vein thrombosis. Ulcer size and duration are also important determinants of healing (2).
Transplantation with a split-thickness skin graft (STSG) is a common intervention in cases of slow-healing VLUs (3–6). The STSG procedure is resource-demanding, and the total cost of each procedure in Denmark is approximately DKK 51,000 (€ 6,800). Thus, a predictive biomarker would be of extreme value to avoid unnecessary surgery with accompanying risks of complications.
The production rate, colour, clarity, viscosity and odour of wound fluid are clinical characteristics of the healing status (7). The molecular composition of the wound fluid has attracted further interest (8, 9). Biomarkers of healing, including cytokines and proteinases, have been evaluated. Furthermore, the bioactivity of wound fluids has been monitored successfully as a proxy for wound healing capacity using various indicator cells (9–11). Different methods of wound fluid collection have been applied to VLUs and normal acute wounds (9). Thus, there is an urgent need for reference concentrations of important molecules in wound fluids collected under standardized and uniform conditions from VLUs and acute wounds. We have developed and validated a method using a retentive hydrophobic foam (9).
The dominant proteinases in wound healing are matrix metalloproteinases (MMPs) and serine proteinases (12). MMP-9 is believed to enhance angiogenesis (13, 14) and MMP-8 may influence the innate immune system (15). Secreted pro MMPs are catalytically inactive. The extracellular activation of latent, pro MMPs is accompanied by the loss of their propeptide (~10 kDa). In addition, the proteolytic activity of MMPs is tightly regulated by tissue inhibitor of metalloproteinases (TIMPs). Increased levels of MMPs have been associated with delayed healing and have been suggested as predictive biomarkers (16). The serine proteinase, human neutrophil elastase (HNE), is secreted by polymorphonuclear neutrophils (PMNs), and its activity is an indicator of PMN tissue infiltration (17). Elevated proteinase activity, as assessed by a bedside test (Woundchek®; Woundchek Laboratories, Fall River, MA, USA), has been shown to predict the take of acellular dermal substitutes in revised diabetic foot ulcers (18). The prediction of autograft healing in VLUs using the diagnostic test has not been studied.
The primary aim of this study was to examine possible biomarkers of the outcome of STSG transplantation of VLUs. Specifically, the levels of inflammatory cell mediators (lipopolysaccharide (LPS) and tumour necrosis factor (TNF)-α) and markers (HNE and myeloperoxidase (MPO)), the levels of MMP-2, MMP-9, MMP-8 and TIMP-1, MMP enzymatic activities, and bioactivities were determined in wound fluids from patients with VLUs and were correlated with STSG outcomes. A secondary aim of this study was to analyse wound fluids obtained by the same wound fluid collection method from STSG donor-site wounds in another cohort of patients with VLUs (19, 20).
Materials and Methods
Ethics and patients
The studies were approved by the Committees of Health Research Ethics in the Capital Region of Denmark (H-16021804 and H-KF-311969). Patients were recruited from the Copenhagen Wound Healing Center, Bispebjerg Hospital, University of Copenhagen, Copenhagen, Denmark, who were scheduled for revision and STSG transplantation of their large and slow-healing non-ischaemic VLUs. None of the patients have received venous surgery. All patiens have received compression therapy as part of the standard therapy. The participants were enrolled if they were over 18 years of age, able to speak and read Danish, and able to give oral and written informed consent. Lactating or pregnant women were excluded.
Venous leg ulcer evaluation and wound fluid procurement
The VLU surface area was determined by digital planimetry from a tracing of the epidermal edge on transparent plastic foil. The extent of the surface populated by granulation tissue was assessed visually by one of the authors (KKM) on a continuous scale from 0% to 100%.
The wound was rinsed gently with water and was not revised immediately prior to application of hydrophobic and sterile collecting polyurethane foam (Fig. 1), covered with a polyurethane film dressing (Tegaderm, 3M, St Paul, MN, USA) and a long-stretch compression bandage according to a validated method (9). After 24 h, the accumulated wound fluid was squeezed out of the foam, centrifuged for 5 min at 500 ×g and sterile-filtered (0.22 µm; Sterivex™, Millipore, Darmstadt, Germany). Wound fluids were aliquoted into Eppendorf polypropylene tubes and stored at –80°C until analysis. None of the patients received local antimicrobial treatments within 1 week of wound fluid sampling.
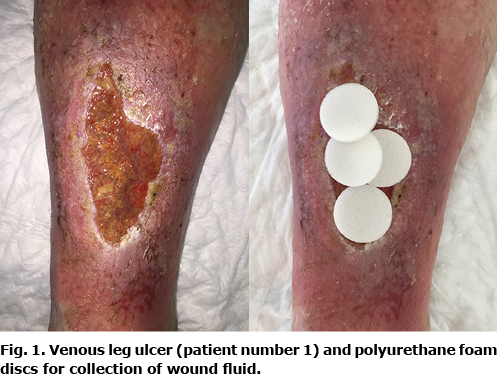
Harvest, transplantation, and healing of split-thickness skin graft in venous leg ulcers
Following wound fluid collection, the donor skin and the skin surrounding the VLU were disinfected with 70% ethanol containing 2% chlorhexidine gluconate. Cefuroxime (i.v.) was given peroperatively. The donor STSG (0.03 cm thick) was harvested from the ipsilateral thigh with an air-driven dermatome (Zimmer Biomet, Warsaw, IN, USA) and meshed with an expansion ratio of 1:1.5 (Meshgraft™, Zimmer Biomet) (21). The wound tissue of the recipient VLU was excised using a flexible surgical blade (BiopBlade®, Integra Life Sciences Corporation, Plainsboro, NJ, USA) parallel to the wound surface. The meshed STSG was applied to the debrided recipient VLU according to our standard procedure (3).
Patients were followed up in the hospital at postoperative weeks 1, 6 and 12.
Høgsberg et al. (4) defined successful healing as more than 98% complete healing of the STSG after 12 weeks, while less healing was considered to be non-healing. Outcomes at 12 weeks correspond well with long-term healing (4). We adopted this dichotomous scale and defined a good outcome as ≥ 98% graft healing (no defect = 0 or minor defect = 1) and a poor outcome as < 98% healing (major defect = 3 or total loss of graft = 4). This information was obtained from the records at 12 weeks postoperatively by 1 author (KKM).
Total protein determination
Total protein was determined using the Pierce™ BCA Protein Assay Kit (Thermo Fisher Scientific). Samples and the bovine serum albumin (BSA) standard were diluted in phosphate-buffered saline (PBS) and the assay was run according to the manufacturer’s recommendations. Absorbances at 562 nm and 690 nm were used for concentration calculations.
Enzyme-linked immunosorbent assay measurements
Enzyme-linked immunosorbent assay (ELISA) kits from RayBiotech (Norcross, GA, USA) were used to determine TNF-α (ELH-TNFA), total (pro and active forms) MMP-2 (ELH-MMP2), total (pro and active forms) MMP-9 (ELH-MMP9) and total (pro and active forms) MMP-8 (ELH-MMP8). TIMP-1 (ab18793) was determined by a kit from Abcam (Cambridge, UK), and LPS (MBS702450) was determined by a kit from MyBioSource (San Diego, CA, USA). Samples were analysed in duplicate.
Western blot analysis of matrix metalloproteinase-8
Samples were separated on Criterion XT Precast 26-well 4–12% SDS-PAGE gradient gels at 30 V for 10 min and then at 180 V for 1 h, and electrotransferred at 100 V for 1 h at 4°C onto a nitrocellulose membrane (Bio-Rad, Hercules, CA, USA). The nitrocellulose membrane was first stained with Ponceau S (0.1%, w/v) in 1% acetic acid for 6 min at ambient temperature, photographed, washed thoroughly in distilled water (dH2O), blocked with an Odyssey buffer, incubated for 18 h at 4°C with monoclonal rabbit antibody against human MMP-8 (ab81286; Abcam Cambridge, UK) diluted 1:1000, and then with goat anti-rabbit DyLight® (Thermo Fisher Scientific, Waltham, MA, USA) 800-conjugated secondary antibody diluted 1:5000 for 1 h at ambient temperature (22). The immunoreactions were visualized using infrared imaging and band intensities were estimated by densitometry (ImageJ, NIH, Bethesda, MD, USA). The amount of pro MMP-8 and active MMP-8 molecular species were estimated by comparison with the intensity of rhMMP-8 (908-MP, R&D Systems, Minneapolis, MN, USA). The proportion of active MMP-8 to total MMP-8 (sum of pro and active MMP-8) was calculated and expressed as a percentage. The Western blot analysis was repeated 3 times.
Enzyme activity assays
Wound fluid samples were diluted in PBS. At least 2 different dilutions of each sample were used for the determination of activity. Fluorescence and absorbance were measured using an Infinite® M200 Pro microplate reader (Tecan, Männedorf, Switzerland). Data were analysed by the Magellan™ 7.2 software.
Human neutrophil elastase. Samples were incubated for 1 h at ambient temperature with 700 pmol/ml substrate V (MeOSuc-Ala-Ala-Pro-Val-7-amino-4-methylcoumarin; 324740, Sigma–Aldrich, St Louis, MO, USA) in assay buffer (600 mM NaCl, 0.05% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate hydrate, 50 mM Tris, pH 7.4) in a 384-well black polystyrene plate. A standard curve (0.08–5 pmol/ml) was generated from elastase from human leukocytes (E8140, Sigma–Aldrich). Fluorescence was measured at excitation 380 nm and emission 460 nm.
Myeloperoxidase. Samples were incubated for up to 20 min at 37°C with an equal volume of 3,3’,5,5’-tetramethylbenzidine (00–4201–56, Thermo Fisher Scientific) in assay buffer (0.5% hexadecyltrimethylammonium bromide, 78 mM NaH2PO4·H2O, 1.67 mM Na2HPO4, pH 5.40) in a transparent 384-well plate. MPO from human leukocytes (M6908, Sigma–Aldrich) served as a standard (2–120 mU/ml). The reaction was stopped by the addition of 2 N H2SO4 and the absorbance was measured at 450 nm.
Matrix metalloproteinase. Samples were incubated for 2 h at 37°C with 5,000 pmol/ml of the substrate 7-methoxycoumarin-4-acetic acid-Lys-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 (Biosyntan, Berlin, Germany) in assay buffer (100 mM NaCl, 10 mM CaCl2, 10 µM ZnCl2, 0.075% (v/v) Brij-35, 100 mM Tris, pH 7.4) in a 384-well black polystyrene plate. Native and active MMP-9 isolated from human PMNs (ab168863, Abcam) was used to generate the standard curve (0.16–10 pmol/ml). Fluorescence was measured at excitation 323 nm and emission 382 nm.
Proteinase point-of-care diagnostic test (Woundchek®)
Wound fluid samples (30 µl) were mixed with 130 µl reagent by rotating the dry swab 5 times clockwise in the test card, which was incubated for 10 min and then closed for an additional 5 min incubation at ambient temperature. The colour intensity of the test line was read by the naked eye and compared with the reference line. The reaction was classified either as elevated levels of inflammatory proteinase activity or low levels of inflammatory proteinase activity (23).
Cellular proliferation assay
HaCaT keratinocytes were a gift from N. Fusenig (24). Primary pooled juvenile human dermal fibroblasts (HDFs) were from CELLnTEC (Bern, Switzerland) and were cultured in Dulbecco’s modified Eagle’s medium (DMEM; L0064, Biowest, Nuaillé, France) with low glucose, sodium pyruvate and 2 mM glutamine supplemented with 10% FCS (Gibco), 100 U/ml penicillin and 100 µg/ml streptomycin. Adult human dermal microvascular endothelial cells (HDVECs) were purchased from Lonza (Basel, Switzerland) and cultured in endothelial cell growth medium containing 5% FCS, hydrocortisone, hEGF, hFGF-2, VEGF, R3-IGF-1, ascorbic acid, 30 µg/ml gentamycin and 15 ng/ml amphotericin-B (CC-3202, Lonza).
Cells were seeded in 15 µl medium at 2.5×103 HaCaT keratinocytes (passage: 56)/HDFs (passage: 11) per well or at 3.5×103 HDVECs (passage: 7) per well into 384-well tissue culture plates. Wound fluids were diluted in medium to 16% (v/v), sterile-filtered and 15 µl was added to a final volume of 30 µl. Control cells were treated with 30 µl medium alone. Cells were incubated for 72 h at 37°C in humidified 5% CO2/air, fixed with 4% paraformaldehyde for 15 min at room temperature and washed with PBS. Total cellular protein was determined as a measure of cell number by staining the fixed cells with 0.4% sulforhodamine B (S1402, Sigma–Aldrich) in 1% acetic acid for 30 min (25). The wells were washed with 1% acetic acid until the wash solution was colourless. After drying, the dye was eluted with 10 mM Tris-HCl, pH 7.5 and absorbance at 550 nm was measured. Three wells per sample were assayed.
Blinding
All wound fluid samples were analysed in the same run for each assay without prior knowledge of the origin of the samples.
Statistical analyses
Continuous data were analysed with 2-sided t-test with Welch’s correction. A contingency test (healing outcome) was evaluated with a 2-sided Fisher’s exact test. Relationships between 2 biomarkers were assessed by Pearson correlation coefficients and between Woundchek® outcome and MMP/HNE activities by point-biserial correlation coefficient. The statistical analyses were performed using SPSS Statistics 28.0 software (IBM, Armonk, NY, USA). The significance level was set at p < 0.05, and values are displayed as the mean ± standard error of the mean (SEM).
Results
Seventeen consecutive patients (7 males and 10 females), with a mean age of (SEM) 73 ± 3.2 years and scheduled for STSG transplantation of their VLU, were enrolled from May 2016 to August 2018. The VLUs had a mean size of (SEM) 30 ± 4.3 cm2, contained granulation tissue to varying degrees, and none were clinically infected (Table I).
The 10 patients (3 males and 7 females) with STSG donor-site wounds had a mean age of (SEM) 66 ± 4.4 years (19, 20). The sterile STSG donor-site wounds had the mean size of 103 ± 19 cm2, and were epithelialized to a mean of 0% at postoperative day 2 and to a mean of 30 ± 8.2% at postoperative day 6 (19, 20).
Split-thickness skin graft healing outcome and its correlation with demography, ulcer characteristics, biomarkers, Woundchek® and bioactivities
Ten (59%) of the STSGs showed good STSG healing, and 7 (41%) showed poor STSG healing 12 weeks after transplantation to the VLUs (Table I).
Sex or age had no significant impact on the STSG healing. Neither VLU duration nor size or degree of granulation differed between patients with good and poor STSG healing (Table II).
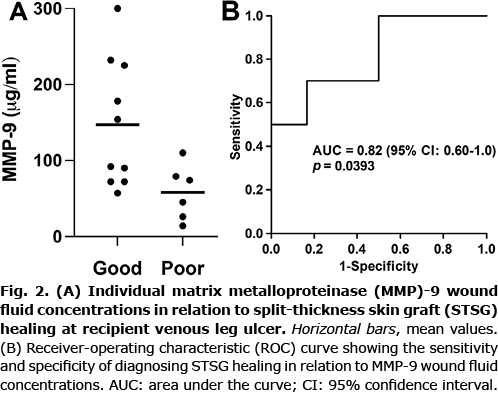
The concentrations of the wound fluid biomarkers with regards to STSG healing are presented in Table II. We found an elevated MMP-9 concentration (estimated difference 89 µg/ml, 95% confidence interval (95% CI) 24–155 µg/ml, p = 0.0116) in wound fluids from VLUs in patients with a good STSG healing outcome compared with those with a poor STSG outcome (Fig. 2A). The diagnostic value (area under the receiver-operating characteristic (ROC) curve) of MMP-9 was 0.82 (95% CI 0.60–1.0, p = 0.0393) from the ROC analysis (Fig. 2B). From this curve a cut-off value of 84 µg/ml with a sensitivity=70% and specificity=84% was derived using the rule of Youden (26). Biomarker levels expressed to the protein content of the wound fluids are presented as median values (interquartile range) and analysed by non-parametric test in Appendix S1; STable I. ROC curve analysis using MMP-9 concentration normalized to the protein content of the wound fluids is shown in Appendix S1 (SFig. 1). These analyses did not change the conclusions.
The point-of-care diagnostic test (Woundchek®) readings correlated with HNE (r = 0.466) and MMP (r = 0.339) activities (Table II). The HNE and MMP activities were determined using the substrates described by Serena et al. (27).
Wound fluids from VLUs in patients with good STSG outcomes showed less inhibition of proliferation of HaCaT keratinocytes than wound fluids from VLUs with poor STSG outcome. There were no significant differences in the other biomarkers regarding the STSG outcome (Table II).
Correlations between biomarkers and bioactivities in venous leg ulcer wound fluids
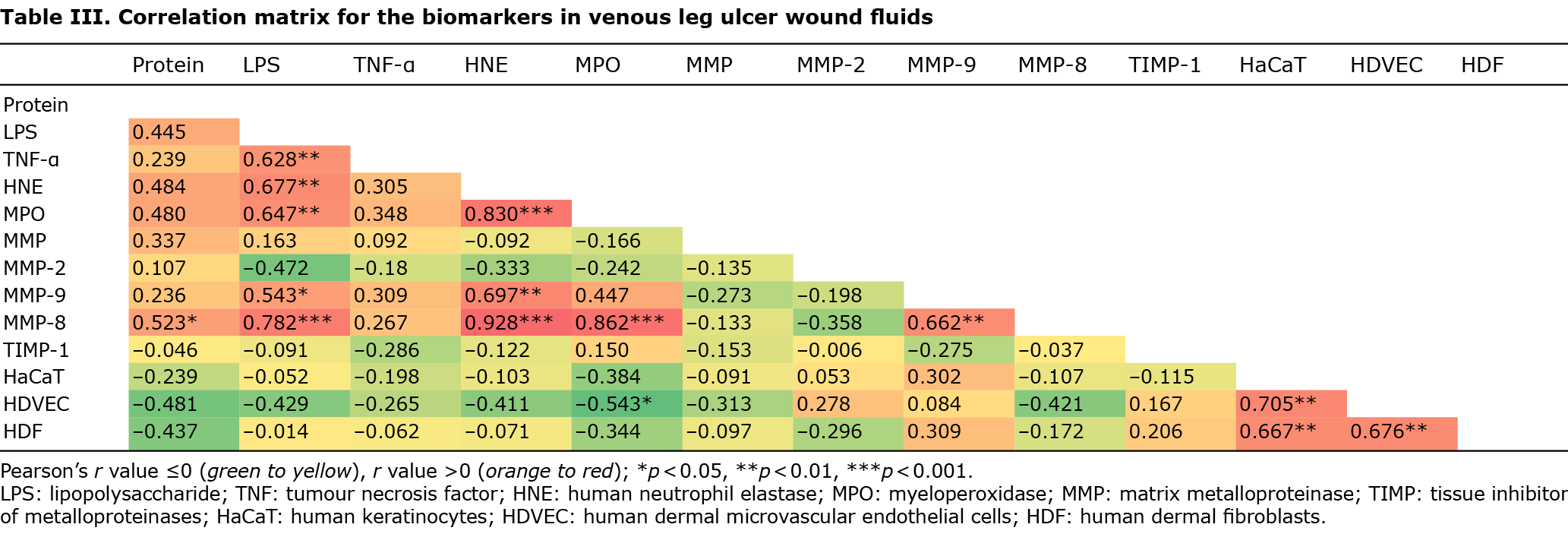
TNF-α, HNE and MPO correlated positively with LPS, and MPO with HNE. MMP-9 and MMP-8 correlated significantly with LPS and HNE, and MMP-8 with protein, MPO and MMP-9. HDVEC proliferation correlated negatively with the inflammatory cell biomarker MPO. Cellular proliferation of the 3 cell types correlated with each other (Table III).
Biomarkers and bioactivities in venous leg ulcers vs split-thickness skin graft donor-site wounds
To confirm that the included patients were representative of VLUs, wound fluids from 10 STSG donor sites were analysed in parallel with a previous study serving as the control for acute wounds (19, 20). These acute wound fluids were collected identically to the chronic wound fluids in the current study (Table IV).
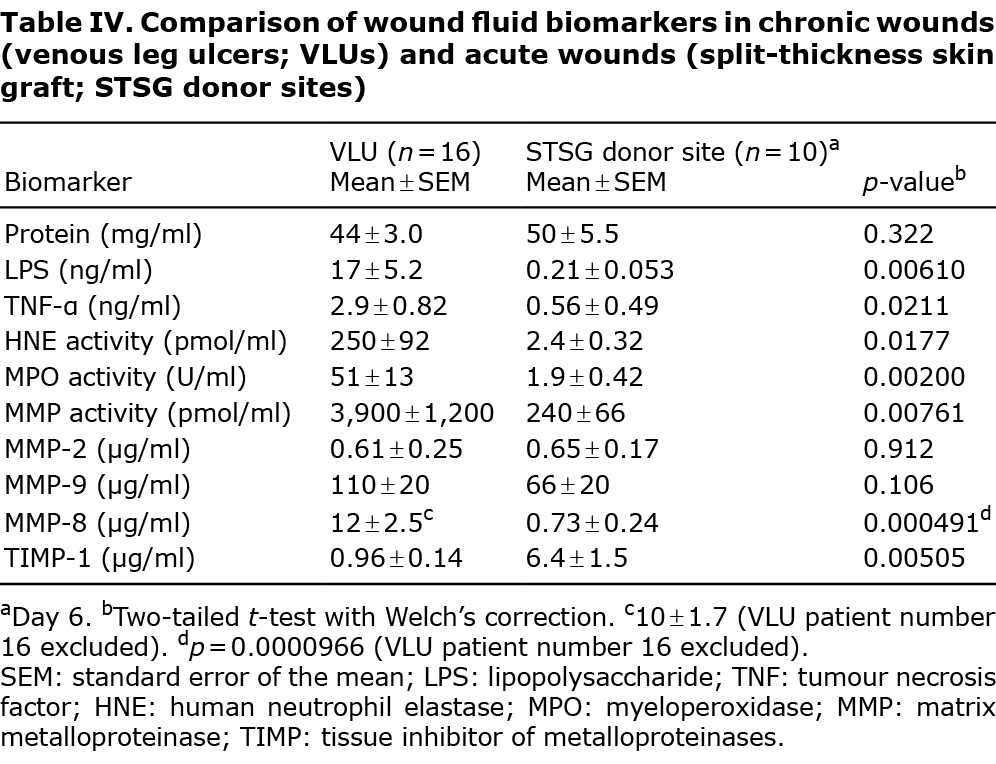
LPS and TNF-α levels increased in VLU wound fluids compared with those in acute wound fluids. The inflammatory cell markers HNE and MPO were elevated in chronic vs acute wound fluids. Overall MMP activity was higher in VLU wound fluids than in STSG donor site wound fluid. Neither MMP-2 nor MMP-9 wound fluid levels differed significantly between chronic and acute wounds. MMP-8 levels were elevated, and TIMP-1 levels were reduced in the VLUs (Table IV).
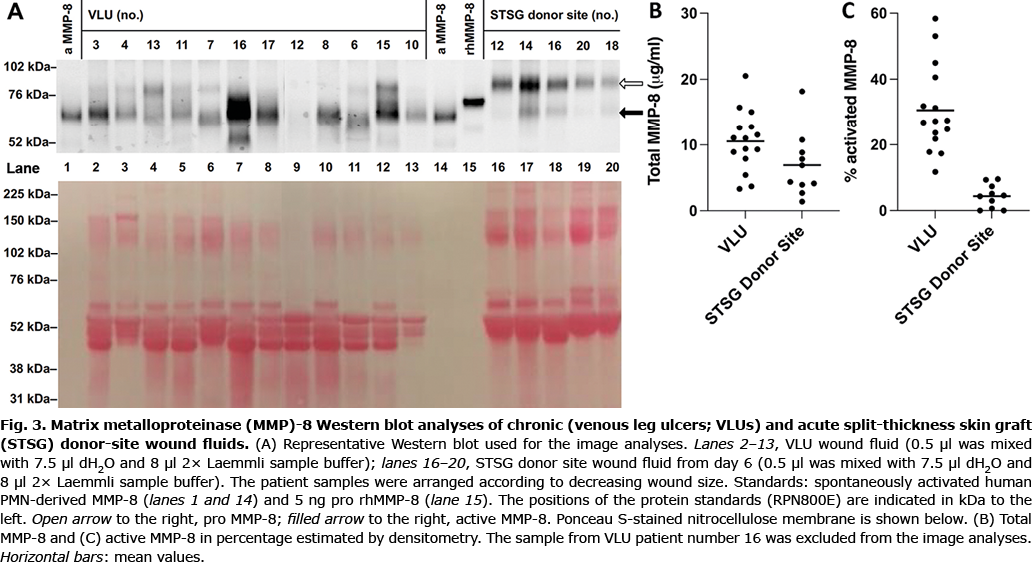
To determine the molecular forms of MMP-8 present in the wound fluids Western immunoblotting analysis was performed (22). A representative Western blot is shown in Fig. 3A. Two distinct bands dominated in the Western blots. These bands most likely represent the pro (open arrow in Fig. 3A) and active (filled arrow in Fig. 3A) forms of PMN-derived MMP-8 (22, 28). The faint band below active MMP-8 at ~50 kDa may originate from fibroblasts (28). In VLU patient number 16 (lane 7 in Fig. 3A), the pattern deviated from the other VLUs by a prominent band at the position of active MMP-8. This band may be the result of reaction between the monoclonal antibody and Staphylococcus aureus products (22). The total amount of immunoreactive MMP-8 was semi-quantified by image analysis as 11 ± 1.2 µg/ml in the VLU group compared with 6.9 ± 1.6 µg/ml in the STSG donor-site wound group (Fig. 3B); this was not statistically significant (p = 0.0784). Proportionally more (p < 0.0001) of the active MMP-8 form was found in VLU (31 ± 3.4%) wound fluids compared with that in STSG donor site (4.4 ± 1.1%) wound fluids (Fig. 3C).
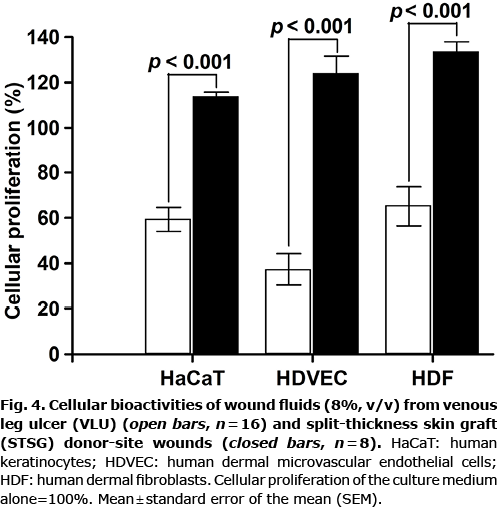
To investigate the overall effect of all factors present in wound fluids, the cell types crucial for wound healing, keratinocytes (HaCaT), endothelial cells (HDVECs) and dermal fibroblasts (HDFs), were exposed to various wound fluid samples. Cellular proliferation to VLU wound fluids was reduced for the 3 cell types compared with wound fluids collected from STSG donor-site wounds (Fig. 4).
Correlation of matrix metalloproteinase activity with inflammatory cell markers and healing of split-thickness skin graft donor-site wounds
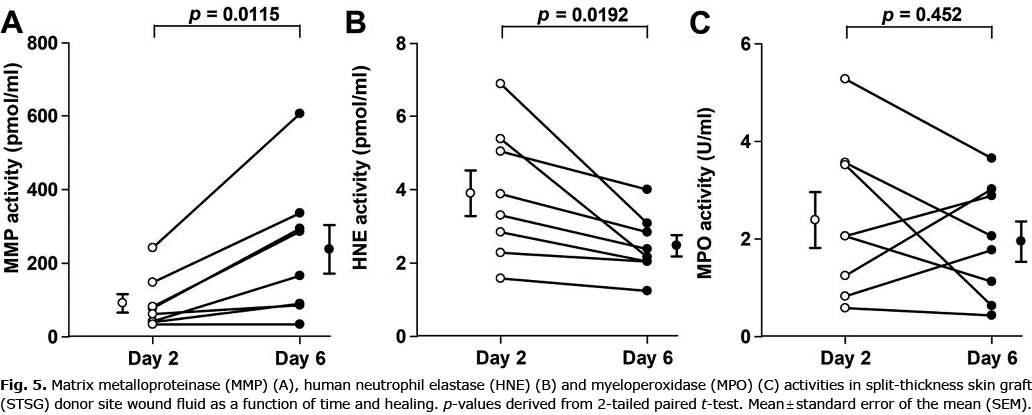
To study the variations in MMP activity and inflammatory cell involvement with healing of acute wounds, sterile wound fluids (negative bacterial swabs) were analysed on postoperative days 2 and 6 (19). MMP activity increased and HNE activity decreased with healing from postoperative days 2 to 6. The inflammatory activity measured by MPO activity did not change in this interval (Fig. 5).
Discussion
STSG transplantation is a common, but resource- demanding, procedure for slow-healing VLUs. A bedside evaluation tool may aid clinicians in deciding whether these patients are unsuitable for skin transplantation and avoid exposing them to unnecessary costly surgery and hospitalization. The major finding of this exploratory study was the possible association between good STSG healing and an increased total MMP-9 concentration in the extracellular wound fluid collected from VLUs prior to transplantation. The ROC analysis indicated that the MMP-9 wound fluid concentration predicts STSG outcome well (26). The current study did not determine the proportion of activated MMP-9; therefore, it cannot be excluded that these molecular forms of MMP-9 may be more discriminatory than total MMP-9 (20).
The lack of significant differences in overall MMP or HNE activities between good and poor STSG healing appeared to be verified by the diagnostic point-of-care device (Woundchek®), which measures the sum of MMP-8, MMP-9 and HNE activities (23). It should be noted that the qualitative point-of-care device is validated only for use with direct wound swab samples, and it is possible that the 24-h collection period and freezing of wound fluid resulted in some false-positive test results (29).
At first glance, these findings appear counterintuitive, as elevated MMP activities are considered detrimental to VLU wound healing (8, 20, 30). Contrary to this popular opinion, Meyer et al. (31) reported similar initial total MMP activities in VLUs that subsequently healed and those that did not after 12 months. MMPs and MMP-9, in particular, are indispensable for physiological wound healing, and specifically epithelialization, as demonstrated by using general MMP inhibitors and wounded MMP-9-deficient mice (32–34). Analogously, we observed increased MMP activity with healing of the STSG donor-site wound. Weckroth et al. (35) reported increases in MMP-9 levels and activities in wound fluid collected from STSG donor-site wounds with postoperative time. The reverse development was found for PMN infiltration (17), which decreased with time, as would be expected for an acute inflammatory reaction (36). Therefore, the increase in MMP activity was unlikely to be PMN-dependent in the healing wounds. Vogt et al. (37) demonstrated increased levels of interleukin (IL)-1α in wound fluid during the healing stage of STSG donor sites; IL-1α has been shown to activate MMP-9 in human skin (38). Thus, increased MMP activity and MMP-9 may reflect increased keratinocyte activity during epithelialization (30, 39, 40). In support of this was the seemingly higher proliferative activity of wound fluid from VLUs with good vs poor STSG healing on keratinocytes. In addition, MMP-9 is proangiogenic (13, 14). Taken together, increases in MMP-9 concentration in wound fluid may indicate improved conditions for epithelialization, and possibly for angiogenesis.
In VLUs, MMP-9 levels correlated with the PMN marker HNE, which was increased in the VLUs compared with in STSG donor-site wounds. MMP-9 levels were not significantly increased in VLU compared with STSG donor-site wound fluids; a finding that confirms earlier reports (20, 30). This could be explained by secretion of MMP-9 by the numerous keratinocytes in the donor-site wounds (39, 40). In contrast, no significant local secretion of MMP-9 occurred over 24 h in VLUs (9).
MMP-8 levels correlated strongly with HNE, indicating that most of the MMP-8 was PMN-derived. This was confirmed by Western blot analysis as PMN-derived MMP-8 has a higher molecular weight than mesenchymal cell type MMP-8 made by, for example, fibroblasts possibly due to a higher degree of glycosylation (28). On the other hand, total MMP-8 levels were increased when measured by ELISA, but not by Western blot analysis. This was due to increased detection of MMP-8 in STSG donor-site wound fluid. The effect of TIMPs on the analysis results was eliminated in the Western blot analysis, but may suppress the signal in the ELISA setup. TIMP-1 levels were higher in STSG donor-site wound fluid and may explain the increase in measured MMP-8 content in STSG donor-site wound fluid in the Western blot vs the ELISA assay. Interestingly, more active MMP-8 might contribute to increased PMN infiltration in the VLUs by activating certain CXC chemokines (15), although the role of MMP-8 in wound healing is not fully understood (41).
Study limitations
One limitation of the current study was the small-scale panel of biomarkers examined. Unbiased profiling of wound fluids by peptidomic/proteomic technologies would have expanded the panel of candidate biomarkers and possibly improved the predictive ability (42, 43). These technologies could also have been applied to identify the MMPs that contributed, in addition to the low TIMP-1 levels, to the high MMP activity in VLU wound fluids (43).
All wound fluids were collected using the same standardized method (9) and were sterile-filtered to eliminate the effect of microorganisms on the measured analytes. This was crucial to enable comparison, not only within the VLU cohort, but also when comparing with the acute STSG donor-site wounds that were sterile. The drawback may be the loss of individual or aggregated peptides/proteins by the filtration procedure (42).
Due to the exploratory nature of the study, we have not adjusted p-values for multiple testing by, for example, applying the method of Benjamini & Hochberg (44).
Conclusion
We suggest that wound fluid MMP-9 is a promising predictor of healing of STSG transplanted to VLUs. A larger sample size is required to determine the feasibility of using MMP-9 as a predictive biomarker for STSG healing. Furthermore, we demonstrated increased HNE activity, indicating increased PMN infiltration (17) and elevated activity of PMN-derived MMP-8 in VLUs vs in acute wounds.
AcknowledgEments
The authors thank the staff at the Copenhagen Wound Healing Center for their help in recruiting patients and collecting samples.
This work has not been externally funded. Woundchek® was provided by Woundchek Laboratories. Akribes Biomedical GmbH performed the HNE, MPO and MMP assays, and the proliferation assays.
Conflicts of interest: KKM, ON, VP, TK and MSÅ have no conflicts of interests to declare. PD, NS and BWW are employees of Akribes Biomedical GmbH, Vienna, Austria.
References
- Guest JF, Fuller GW, Vowden P. Venous leg ulcer management in clinical practice in the UK: costs and outcomes. Int Wound J 2018; 15: 29–37.
- Melikian R, O’Donnell TF, Jr, Suarez L, Iafrati MD. Risk factors associated with the venous leg ulcer that fails to heal after 1 year of treatment. J Vasc Surg Venous Lymphat Disord 2019; 7: 98–105.
- Bitsch M, Saunte DM, Lohmann M, Holstein PE, Jørgensen B, Gottrup F. Standardised method of surgical treatment of chronic leg ulcers. Scand J Plast Reconstr Surg Hand Surg 2005; 39: 162–169.
- Høgsberg T, Bjarnsholt T, Thomsen JS, Kirketerp-Møller K. Success rate of split-thickness skin grafting of chronic venous leg ulcers depends on the presence of Pseudomonas aeruginosa: a retrospective study. PLoS One 2011; 6: e20492.
- Serra R, Rizzuto A, Rossi A, Perri P, Barbetta A, Abdalla K, et al. Skin grafting for the treatment of chronic leg ulcers – a systematic review in evidence-based medicine. Int Wound J 2017; 14: 149–157.
- Patel BJ, Asher CM, Bystrzonowski N, Healy C. Safeguarding skin grafts: an evidence-based summary of fixation techniques. Ann Plast Surg 2021; 87: e180–e188.
- Atkin L, King B, Duffus-Grovell D, Meagher H, Chaplin S, Davies S. Highly exuding non-healing leg ulcers: a surmountable challenge. Br J Nurs 2021; 30: S3–S20.
- Power G, Moore Z, O’Connor T. Measurement of pH, exudate composition and temperature in wound healing: a systematic review. J Wound Care 2017; 26: 381–397.
- Zillmer R, Trøstrup H, Karlsmark T, Ifversen P, Ågren MS. Duration of wound fluid secretion from chronic venous leg ulcers is critical for interleukin-1α, interleukin-1β, interleukin-8 levels and fibroblast activation. Arch Dermatol Res 2011; 303: 601–606.
- Katz MH, Alvarez AF, Kirsner RS, Eaglstein WH, Falanga V. Human wound fluid from acute wounds stimulates fibroblast and endothelial cell growth. J Am Acad Dermatol 1991; 25: 1054–1058.
- Trengove NJ, Bielefeldt-Ohmann H, Stacey MC. Mitogenic activity and cytokine levels in non-healing and healing chronic leg ulcers. Wound Repair Regen 2000; 8: 13–25.
- Toriseva M, Kähäri VM. Proteinases in cutaneous wound healing. Cell Mol Life Sci 2009; 66: 203–224.
- Gillard JA, Reed MW, Buttle D, Cross SS, Brown NJ. Matrix metalloproteinase activity and immunohistochemical profile of matrix metalloproteinase-2 and -9 and tissue inhibitor of metalloproteinase-1 during human dermal wound healing. Wound Repair Regen 2004; 12: 295–304.
- Ardi VC, Kupriyanova TA, Deryugina EI, Quigley JP. Human neutrophils uniquely release TIMP-free MMP-9 to provide a potent catalytic stimulator of angiogenesis. Proc Natl Acad Sci U S A 2007; 104: 20262–20267.
- Tester AM, Cox JH, Connor AR, Starr AE, Dean RA, Puente XS, et al. LPS responsiveness and neutrophil chemotaxis in vivo require PMN MMP-8 activity. PLoS ONE 2007; 2: e312.
- Westby MJ, Norman G, Watson REB, Cullum NA, Dumville JC. Protease activity as a prognostic factor for wound healing in complex wounds. Wound Repair Regen 2020; 28: 631–644.
- Lammers AM, van de Kerkhof PC, Schalwijk J, Mier PD. Elastase, a marker for neutrophils in skin infiltrates. Br J Dermatol 1986; 115: 181–186.
- Izzo V, Meloni M, Vainieri E, Giurato L, Ruotolo V, Uccioli L. High matrix metalloproteinase levels are associated with dermal graft failure in diabetic foot ulcers. Int J Low Extrem Wounds 2014; 13: 191–196.
- Trøstrup H, Holstein P, Christophersen L, Jorgensen B, Karlsmark T, Høiby N, et al. S100A8/A9 is an important host defence mediator in neuropathic foot ulcers in patients with type 2 diabetes mellitus. Arch Dermatol Res 2016; 308: 347–355.
- Trøstrup H, Holstein P, Karlsmark T, Moser C, Ågren MS. Uncontrolled gelatin degradation in non-healing chronic wounds. J Wound Care 2018; 27: 724–734.
- Danielsen P, Jørgensen B, Karlsmark T, Jorgensen LN, Ågren MS. Effect of topical autologous platelet-rich fibrin versus no intervention on epithelialization of donor sites and meshed split-thickness skin autografts: a randomized clinical trial. Plast Reconstr Surg 2008; 122: 1431–1440.
- Kirketerp-Møller K, Bjarnsholt T, Jensen PO, Ågren MS. Staphylococcus aureus augments release of matrix metalloproteinase-8 from human polymorphonuclear leukocytes. Acta Derm Venereol 2020; 100: adv00232.
- Serena TE. Development of a novel technique to collect proteases from chronic wounds. Adv Wound Care (New Rochelle) 2014; 3: 729–732.
- Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol 1988; 106: 761–771.
- Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, et al. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst 1990; 82: 1107–1112.
- Carter JV, Pan J, Rai SN, Galandiuk S. ROC-ing along: evaluation and interpretation of receiver operating characteristic curves. Surgery 2016; 159: 1638–1645.
- Serena TE, Cullen BM, Bayliff SW, Gibson MC, Carter MJ, Chen L, et al. Defining a new diagnostic assessment parameter for wound care: elevated protease activity, an indicator of nonhealing, for targeted protease-modulating treatment. Wound Repair Regen 2016; 24: 589–595.
- Hanemaaijer R, Sorsa T, Konttinen YT, Ding Y, Sutinen M, Visser H, et al. Matrix metalloproteinase-8 is expressed in rheumatoid synovial fibroblasts and endothelial cells. Regulation by tumor necrosis factor-alpha and doxycycline. J Biol Chem 1997; 272: 31504–31509.
- Lockmann A, Schill T, Hartmann F, Grönemeyer LL, Holzkamp R, Schön MP, et al. Testing elevated protease activity: prospective analysis of 160 wounds. Adv Skin Wound Care 2018; 31: 82–88.
- Mirastschijski U, Impola U, Jahkola T, Karlsmark T, Ågren MS, Saarialho-Kere U. Ectopic localization of matrix metalloproteinase-9 in chronic cutaneous wounds. Hum Pathol 2002; 33: 355–364.
- Meyer FJ, Burnand KG, Abisi S, TeKoppele JM, van Els B, Smith A. Effect of collagen turnover and matrix metalloproteinase activity on healing of venous leg ulcers. Br J Surg 2008; 95: 319–325.
- Ågren MS, Mirastschijski U, Karlsmark T, Saarialho-Kere UK. Topical synthetic inhibitor of matrix metalloproteinases delays epidermal regeneration of human wounds. Exp Dermatol 2001; 10: 337–348.
- Kyriakides TR, Wulsin D, Skokos EA, Fleckman P, Pirrone A, Shipley JM, et al. Mice that lack matrix metalloproteinase-9 display delayed wound healing associated with delayed reepithelization and disordered collagen fibrillogenesis. Matrix Biol 2009; 28: 65–73.
- Hattori N, Mochizuki S, Kishi K, Nakajima T, Takaishi H, D’Armiento J, et al. MMP-13 plays a role in keratinocyte migration, angiogenesis, and contraction in mouse skin wound healing. Am J Pathol 2009; 175: 533–546.
- Weckroth M, Vaheri A, Lauharanta J, Sorsa T, Konttinen YT. Matrix metalloproteinases, gelatinase and collagenase, in chronic leg ulcers. J Invest Dermatol 1996; 106: 1119–1124.
- Kim MH, Liu W, Borjesson DL, Curry FR, Miller LS, Cheung AL, et al. Dynamics of neutrophil infiltration during cutaneous wound healing and infection using fluorescence imaging. J Invest Dermatol 2008; 128: 1812–1820.
- Vogt PM, Lehnhardt M, Wagner D, Jansen V, Krieg M, Steinau HU. Determination of endogenous growth factors in human wound fluid: temporal presence and profiles of secretion. Plast Reconstr Surg 1998; 102: 117–123.
- Han YP, Downey S, Garner WL. Interleukin-1alpha-induced proteolytic activation of metalloproteinase-9 by human skin. Surgery 2005; 138: 932–939.
- Mirastschijski U, Bugdahl R, Rollman O, Johansson BR, Ågren MS. Epithelial regeneration from bioengineered skin explants in culture. Br J Dermatol 2006; 154: 42–49.
- Buisson AC, Zahm JM, Polette M, Pierrot D, Bellon G, Puchelle E, et al. Gelatinase B is involved in the in vitro wound repair of human respiratory epithelium. J Cell Physiol 1996; 166: 413–426.
- Danielsen PL, Holst AV, Maltesen HR, Bassi MR, Holst PJ, Heinemeier KM, et al. Matrix metalloproteinase-8 overexpression prevents proper tissue repair. Surgery 2011; 150: 897–906.
- van der Plas MJ, Cai J, Petrlova J, Saleh K, Kjellström S, Schmidtchen A. Method development and characterisation of the low-molecular-weight peptidome of human wound fluids. Elife 2021; 10: e66876.
- Sabino F, Hermes O, Egli FE, Kockmann T, Schlage P, Croizat P, et al. In vivo assessment of protease dynamics in cutaneous wound healing by degradomics analysis of porcine wound exudates. Mol Cell Proteomics 2015; 14: 354–370.
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B 1995; 57: 289–300.