An increase in incidence of chilblain-like lesions of fingers and toes has been recorded across Europe contemporarily with the peaks of incidence of SARS-CoV-2 infection (1). There are several hypotheses about a role of the virus in the development of these chilblain-like lesions. Some authors have theorized an exaggerated immune response, which is effective against the infectious potential of the virus, but induces vascular injury (1). A role has been proposed for up-regulated interferon type I induced (IFN-) protein, to explain chilblain-like lesions in patients who have tested negative for SARS-CoV-2: since active viral replication is not necessary to mount an efficient IFN response in SARS-CoV-2 infection, inhibition of coronavirus replication by IFN-induced trans-membrane protein and depletion of B cells caused by high expression of IFN-I may explain negative PCR and serological tests, respectively (2). Type I IFN is important in early response to viral infections, but, when this response occurs inappropriately, it can contribute to immune pathologies. Moreover, chilblains are a typical feature of type I interferonopathies (3). Nonetheless, SARS-CoV-2 spike protein was found in capillary endothelial cells of the upper dermis in 7 paediatric patients, leading to the hypothesis of vascular damage induced directly by the virus (4).
In March 2021, 3 patients presented to our clinic reporting development of acral lesions shortly after immunization against COVID-19.
CASE REPORTS
Case one. A 47-year-old woman received one dose of Oxford-AstraZeneca COVID-19 vaccine on 19 February 2021. She noticed erythematous lesions on the skin of her fingers on 27 February 2021. On 6 March 2021, physical examination revealed chilblain-like lesions involving the II, IV and V fingers of her right hand.
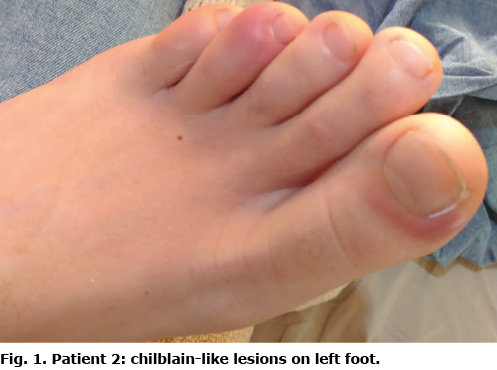
Case two. A 30-year-old woman developed chilblain-like lesions in late February, after having received the second dose of Pfizer-BioNTech COVID-19 vaccine on 4 February 2021. On 4 March 2021, she presented with chilblain-like lesions on the II, III and V fingers of her left hand and I, IV and V toes of her left foot (Fig. 1).
Case three. A 55-year-old woman received the second dose of Pfizer-BioNTech COVID-19 vaccine on 20 February 2021. She noticed skin lesions on her fingers in mid-March. On 20 March 2021 she was evaluated at our clinic due to chilblain-like lesions on the dorsal aspect of the III and V fingers of her right hand.
All patients denied symptoms of SARS-CoV-2 infection and denied contact with infected individuals. All of them had negative results of laboratory investigations: blood cell count was normal, as were creatinine, lipid profile and C-reactive protein (CRP); antinuclear antibodies, antiphospholipid antibodies, antineutrophil cytoplasmic antibodies (ANCA), cryoglobulin, cold agglutinin were all negative. Rapid antigenic SARS-CoV-2 and serological tests were negative. All patients refused biopsies as they reported that the lesions were spontaneously regressing. Interestingly, all patients reported isolated episodes of chilblains during their adolescence; however, chilblains previously occurred only following exposition to very cold temperatures. Conversely, all patients were currently living in temperate areas. In all cases the lesions resolved spontaneously in less than 2 weeks.
DISCUSSION
Currently, a causal association between immunization against COVID-19 and the development of chilblain-like lesions cannot be demonstrated: to date, only a few isolated reports have been published (5–8). A study on an international registry of 803 vaccine reactions reported 3 cases of chilblain-like lesions (9). Notably, all the reactions reported occurred after mRNA vaccinations. Multiple cases of chilblain-like lesions, similar to those previously linked with SARS-CoV-2 infection, occurring 1–4 weeks after immunization make us suspect a relationship with the vaccination. In addition, this appears to support to the hypothesis of an immune-mediated pathogenic mechanism, rather than a direct vascular damage by the virus. It is possible that antibodies against SARS-CoV-2 spike protein, developing after vaccination, could induce endothelial damage in predisposed patients, directly or via deposition of immune complexes. A lymphocytic infiltrate involving blood vessels might have been caused by the vaccination, as demonstrated in COVID-19-related chilblain-like lesions (1).
This case series is of interest because it includes the first report of chilblain-like lesions occurring after an Oxford-AstraZeneca COVID-19 vaccination. It is notable that 2 patients developed the lesions after the second Pfizer-BioNTech dose; only one similar case is reported to date (10).
In conclusion, wider studies are required to ascertain the relationship between immunization against COVID-19 and onset of chilblain-like lesions, taking into consideration the advent of third doses. These case reports are a further indication of an association between SARS-CoV-2 infection and chilblain-like skin lesions.
The authors have no conflicts of interest to declare.
REFERENCES
- Kanitakis J, Lesort C, Danset M, Jullien D. Chilblain-like acral lesions during the COVID-19 pandemic (“COVID toes”): histologic, immunofluorescence, and immunohistochemical study of 17 cases. J Am Acad Dermatol 2020; 83: 870–875.
- Battesti G, Descamps V. Negative tests for SARS-CoV-2 infection do not rule out its responsibility for chilblains. Br J Dermatol 2020; 183: 1151.
- Hubiche T, Cardot-Leccia N, Le Duff F, Seitz-Polski B, Giordana P, Chiaverini C, et al. Clinical, laboratory, and interferon-alpha response characteristics of patients with chilblain-like lesions during the COVID-19 pandemic. JAMA Dermatol 2021; 157: 202–206.
- Colmenero I, Santonja C, Alonso-Riaño M, Noguera-Morel L, Hernández-Martín A, Andina D, et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol 2020; 183: 729–737.
- Lesort C, Kanitakis J, Donzier L, Jullien D. Chilblain-like lesions after BNT162b2 mRNA COVID-19 vaccine: a case report suggesting that ‘COVID toes’ are due to the immune reaction to SARS-CoV-2. J Eur Acad Dermatol Venereol 2021; 35: e630–e632.
- Davido B, Mascitti H, Fortier-Beaulieu M, Jaffal K, de Truchis P. ‘Blue toes’ following vaccination with the BNT162b2 mRNA COVID-19 vaccine. J Travel Med 2021; 28: taab024.
- Meara AS, Silkowski M, Quin K, Jarjour W. A case of chilblains-like lesions post SARS-CoV-2 vaccine? J Rheumatol 2021; 48: 1754.
- Holmes GA, Desai M, Limone B, Love, J, Tawfik M, Wong L, Furukawa B. A case series of cutaneous COVID-19 vaccine reactions at Loma Linda University Department of Dermatology. JAAD Case Rep 2021; 16: 53–57.
- Pileri A, Guglielmo A, Raone B, Patrizi A. Chilblain lesions after COVID-19 mRNA vaccine. Br J Dermatol 2021; 185: e3.
- McMahon DE, Amerson E, Rosenbach M, Lipoff JB, Moustafa D, Tyagi A, et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol 2021; 85: 46–55.
- Cameli N, Silvestri M, Mariano M, Nisticò SP, Cristaudo A. Pernio-like skin lesions after the second dose of Pfizer-BioNTech COVID-19 vaccine. J Eur Acad Dermatol Venereol 2021; 35: e725–e727.