The effectiveness of systemic treatment for Leishmania tropica cutaneous leishmaniasis remains unclear. The purpose of the study is to evaluate the efficacy and safety of systemic treatments for L. tropica cutaneous leishmaniasis. This retrospective study was performed in 114 patients. Systemic treatments included liposomal amphotericin B and sodium stibogluconate. All patients underwent systemic treatment for L. tropica cutaneous leishmaniasis. Favourable treatment responses were recorded in 72.5% and 70.2% of the patients in the liposomal amphotericin B and sodium stibogluconate groups, respectively; 25.3% and 46% of those in the liposomal amphotericin B and sodium stibogluconate groups respectively, experienced at least one adverse effect. Lesions in cartilaginous areas were associated with higher treatment failure. Prior topical or systemic treatment increased the chance of future systemic treatment success. Liposomal amphotericin B was associated with a shorter intravenous treatment duration and better safety profile. Thus, liposomal amphotericin B is the treatment of choice for L. tropica cutaneous leishmaniasis.
Key words: Leishmania tropica; liposomal amphotericin B (L-AmB); sodium stibogluconate; cutaneous leishmaniasis; systemic treatment.
Accepted Mar 1, 2022; Epub ahead of print Mar 1, 2022
Acta Derm Venereol 2022; 102: adv00721.
DOI: 10.2340/actadv.v102.2079
Corr: Michal Solomon, Department of Dermatology, The Chaim Sheba Medical Center, IL-52621 Tel Hashomer, Israel. E-mail: solomondr1@gmail.com
SIGNIFICANCE
The effectiveness of systemic treatment for Leishmania tropica cutaneous leishmaniasis remains unclear. Systemic treatments include liposomal amphotericin B and sodium stibogluconate. A total of 114 patients underwent systemic treatment for Leishmania tropica cutaneous leishmaniasis. Sodium stibogluconate and liposomal amphotericin B seemingly have similar efficacy. Lesions in cartilaginous areas were associated with higher treatment failure. Prior topical or systemic treatment increased the chance of future systemic treatment success. Liposomal amphotericin B was associated with a shorter treatment duration and better safety profile. Thus, liposomal amphotericin B is the treatment of choice for Leishmania tropica cutaneous leishmaniasis.
INTRODUCTION
Cutaneous leishmaniasis (CL) represents a major health threat, with 0.7–1.2 million cases reported annually worldwide (1). The disease is widely distributed globally, with approximately one-third of cases occurring in each of the following 3 regions: the Americas, the Mediterranean basin, and western Asia from the Middle East to Central Asia (2). CL is endemic to Israel and has been attributed almost exclusively to infection with Leishmania major (3, 4). However, over the last 2 decades, CL due to Leishmania tropica has been increasingly reported in several regions of Israel. L. tropica has predominantly anthroponotic transmission, and its vectors have been identified as. Phlebotomus sergenti and Phlebotomus arabicus. However, in Israel, the putative reservoir was found to be Procavia capensis (rock hyrax) (5) a relatively small mammal, resembling a guinea pig, found across Africa and the Middle East. Regarding the manifestation of CL, nodulo-ulcerative skin lesions are formed at the site of the sandfly bite after an incubation period of 3–12 weeks. The disease is self-limiting, usually over a period of 12–24 months or longer, resulting in significant disfigurement and scarring. L. tropica CL heals more slowly and is relatively resistant to treatment, in contrast to L. major CL (6–9). Furthermore, L. tropica may cause leishmania recidivans and, in rare cases, visceral leishmaniasis (10).
Selecting the optimal treatment regimen for individual patients represents a challenge. The guidelines established by the Infectious Diseases Society of America and American Society of Tropical Medicine and Hygiene state that no ideal or universally applicable therapy for CL has been identified and that some therapies/regimens appear highly effective only against specific Leishmania species/strains in certain areas of the world (11). Furthermore, a recent Cochrane review that explored the different treatments of CL concluded that they were difficult to evaluate due to the variability of Leishmania species, the different regimens used, and the inconsistency of the studies’ duration (12).
There are various systemic treatments aimed at reducing the healing time associated with CL; however, thus far, no treatment has been considered the “gold standard.” Old-World leishmaniasis can be treated with topical or systemic therapies. In our institution the indications for systemic rather than intralesional treatment are as follows: topical treatment failure, patient age < 6 years, multiple lesions (> 5), or non-feasibility of intralesional sodium stibogluconate (SSG) injection because of the anatomical location of the lesions (e.g. the face and eyelids) (13). The approach to systemic treatment of CL has changed over the years. In the past, SSG (Pentostam; GlaxoSmithKline, London, UK) was administered as the first-line treatment (14). Currently, liposomal amphotericin B (L-AmB) is used as the first-line treatment because of its shorter duration and fewer adverse effects (13, 15, 16). However, the intravenous (IV) L-AmB treatment is still used as a rescue treatment in resistant SSG cases. Miltefosine is a new emerging attractive alternative as an oral medication that can be taken at home with good tolerance (17). However, data regarding the efficacy and safety of L-AmB for the treatment of L. tropica CL are scarce and the 2 systemic treatments (L-AmB and SSG) have been compared in only a small group of patients (10, 13). Therefore, this study was conducted in a large sample with the aim of comparing the efficacy of, and adverse events following, the 2 treatments, in order to determine the preferred therapy.
METHODS
This was a retrospective study conducted among patients with L. tropica CL. The patient data was collected from the electronic medical records of the dermatology department and dermatology outpatient clinics and the Center for Geographic Medicine at the Sheba Medical Center, Israel, for the years 2008 to 2020. L. tropica CL was defined as cutaneous lesions (ulcers, nodules, or papules) clinically compatible with leishmaniasis and a polymerase chain reaction assay positive for L. tropica (18) or a smear or biopsy specimen positive for Leishmania amastigotes in a patient residing in a region to which only L. tropica is endemic.
The treatments were administered in the outpatient setting. The protocol for IV L-AmB included a dose of 3–5 mg/kg daily for 5 consecutive days, with a sixth dose administered on day 10. Laboratory parameters were monitored daily and included complete blood cell count and measurement of electrolytes, including magnesium, and liver and kidney function tests. The treatment protocol for IV SSG included a dose of 20 mg kg−1 d−1 for 20 days. During IV SSG treatment, blood counts, liver and kidney function, amylase, lipase, and electrocardiography (ECG) were monitored daily and 1 month after the end of treatment.
In both treatment protocols, when there was a partial response at the end of the treatment, we prolonged the days of the treatment: L-AmB (AmBisome) treatment for up to 10 days, and IV SSG treatment for up to 30 days. Clinical responses were categorized as follows: (i) complete response, defined as 100% re-epithelialization of the ulcer (regression of the lesion in the case of non- ulcerative lesions) within 3 months following cessation of treatment; (ii) partial response, defined as > 50% re-epithelialization of the ulcer within the same period; and (iii) treatment failure, defined as ≤ 50% re-epithelialization of the ulcer within the same period.
Achievement of complete or partial response within 3 months, but a relapse during the follow-up period, was also considered as treatment failure. Patients were followed up every 3–4 weeks until they were completely cured. The study was approved by the institutional review board (protocol approval number 7274-09).
Statistical analysis
Statistical Package for the Social Sciences (SPSS) version 23.0 for Windows software was used for the data entry and analysis. Continuous variables were expressed as the median and interquartile range (IQR) and categorical variables as a percentage.
Fischer’s exact test (2-tailed) was used to compute p-value in the prevalence assessment. A p-value < 0.05 was considered significant.
RESULTS
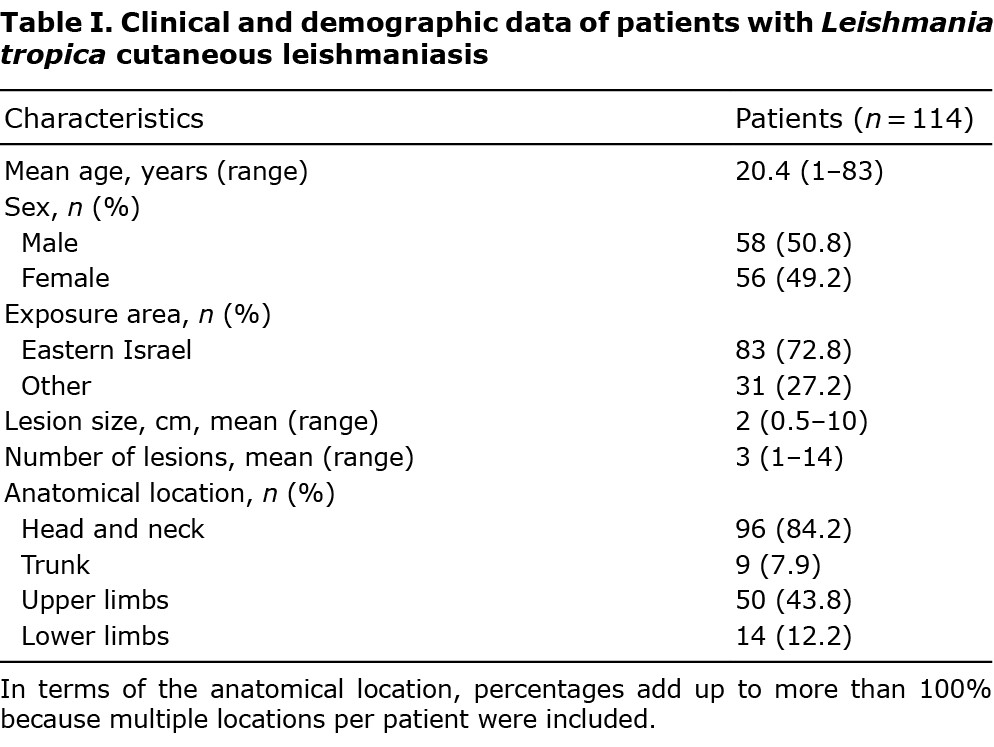
In all, 114 patients underwent systemic treatment for L. tropica CL in our centre between 2008 and 2020. Patients’ clinical and demographic data are shown in Table I. Fifty-eight patients (50.8%) were male; most of the patients were children (60.5%), and the mean age at diagnosis was 20.4 years (range 1–83 years; median 11 years). The mean number of lesions per patient was 3 (range 1–14), and the mean lesion size was 2 cm (range 0.5–10 cm). The lesions were commonly located on the face (96 patients, 84.2%), including the nose (27 patients, 28%) and lips (39 patients, 40.6%), and upper limbs (50 patients, 43.8%) (Table I). The number of body areas involved was 1 in 63 patients (56%) and 2 or more in 51 patients (44%). PCR for L. tropica was positive in 52 patients. All other infections were acquired in regions of Israel to which L. tropica was endemic. None of the patients had significant comorbidities.
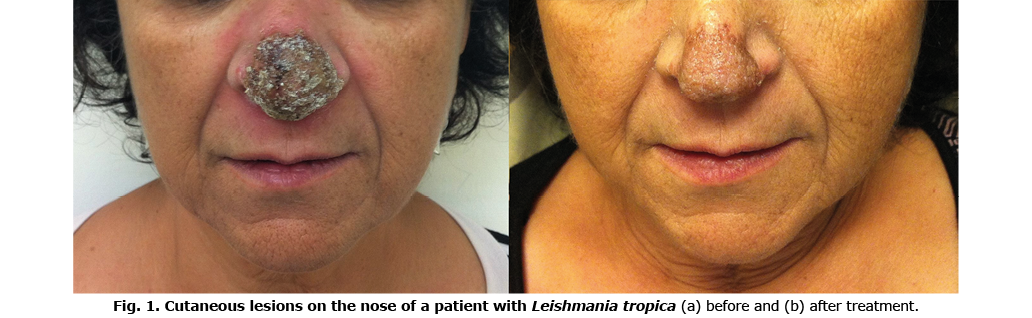
Representative pictures of cutaneous lesions before and after treatment are shown in Fig. 1.
One or more topical therapies failed as first-line treatment in 41 (36%) patients. Topical treatment included paromomycin ointment (n = 18), intralesional SSG (n = 28), cryotherapy (n = 3), or amphotericin 4% topical gel (n = 2). A comparison of the 2 systemic treatments is shown in Table II. Eighty patients received IV L-AmB, of whom 3 showed IV SSG failure. Forty-seven patients received IV SSG, of whom 14 showed IV L-AmB failure. Intergroup differences in age, sex, time to systemic treatment, number of lesions, failure of previous treatments, facial lesions, and location of lesions in cartilaginous areas were not significant. Among the L-AmB-treated patients, 48 (60%) and 10 (12.5%) achieved complete and partial responses, respectively, and 22 (27.5%) showed treatment failures. Among the patients treated with IV SSG, 30 (63.8%) and 3 (6.3%) achieved complete and partial responses, respectively, and 14 (29.8%) showed treatment failure.

Adverse effects were observed in 21 (25.3%) and 23 (46%) patients treated with L-AmB and IV SSG, respectively (p = 0.013). The adverse effects of L-AmB treatment included renal function abnormalities and nausea. Three (3.6%) patients discontinued treatment due to allergic reactions. In the IV SSG group, the adverse effects included nausea and vomiting, hyperamylasaemia, and increased parameters in the liver function test. Three patients (6.3%) discontinued treatment due to adverse effects, including QT prolongation on ECG in 1 patient and liver and pancreatic enzyme elevation in 2 patients.
The treatment period was significantly longer in the IV SSG group than in the L-AmB group (18 days (range 1–60 days) vs 6.3 days (range 1–15 days), p < 0.001). Altogether, 18 patients received treatment for a period longer than that of the standard regimen, either due to adverse effects that required a reduction in the daily dose and extension of treatment duration to achieve the desired total dose or standard treatment failure and increase in the number of treatment days to achieve the desired result. For the 10 patients in the L-AmB group for whom treatment was extended, the mean treatment duration was 8 days instead of 6 days (133% extension of treatment) at a cumulative dose of 18–40 mg/kg. On the other hand, for the 8 patients in the IV SSG group for whom treatment was extended, the mean treatment duration was 27 days (135% extension of treatment) at a cumulative dose of 400–600 mg/kg.
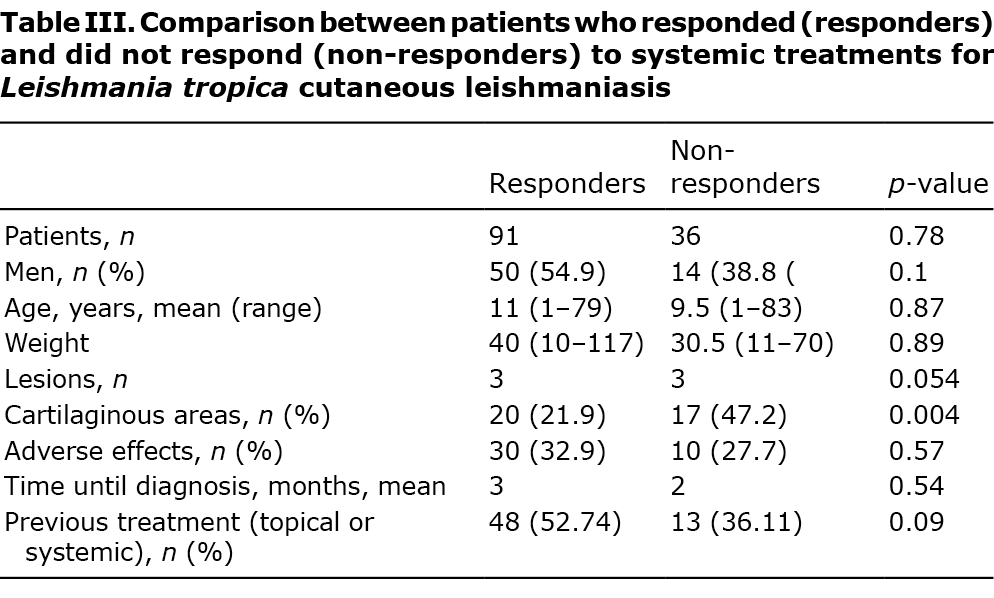
Comparison of those who showed a treatment response and treatment failure revealed no difference between the groups in terms of sex, age, weight, number of lesions, time to treatment, or presence of adverse effects (Table III). However, lesions in areas involving the cartilage were more common in the treatment failure group. Regression analysis validated the increased treatment failure in patients with lesions in cartilaginous areas (odds ratio [OR] 0.244, p = 0.004, 95% confidence interval (95% CI) 0.093–0.641). In contrast, prior topical or systemic treatment, regardless of whether it had failed, increased the chance of systemic treatment success (OR 4.211, p = 0.01, 95% CI 1.416–12.522).
DISCUSSION
Most studies of Old-World CL from countries to which both L. tropica and L. major are endemic did not include speciation. The optimal treatment regimen for L. tropica CL remains to be determined. Treatment failure may be associated with host factors (such as immune status or lesion location) or drug resistance of the parasite (19). This retrospective study conducted in a large sample of patients compared the efficacy and adverse events following 2 systemic treatments for L. tropica CL to determine the preferred therapy. We compared 2 treatments, IV SSG and IV L-AmB, in 127 treatment trials and found similar response rates (72.5% and 70.2% for L-AmB and SSG, respectively, p = 0.78). However, IV SSG treatment was associated with a higher rate of adverse effects (22.5% and 42.5% for L-AmB and SSG, respectively, p = 0.019) and a longer treatment period per protocol. Furthermore, we found the involvement of cartilaginous areas was associated with significantly higher treatment failure. In contrast, a previous topical or systemic treatment course was associated with an improved response rate (p = 0.01).
The IV SSG failure rate in the current study was 29.8%, which was higher than that reported in previous studies on the antimoniate treatment of L. tropica CL (15). The failure rate among patients who received meglumine antimoniate (Glucantime) intramuscular injections (20 mg/kg for 20 days) in Iran was 9.8–12% (20, 21). This may suggest that the L. tropica species in Israel are more resistant compared with those in other parts of the world, including the Middle East. Unresponsiveness to meglumine antimoniate (Glucantime) treatment was reported in cases of L. tropica CL and not L. major CL in Iran, and treatment failure was associated with primary treatment resistance of L. tropica (20, 21). Several mechanisms have been proposed for the treatment resistance of L. tropica, including downregulation of the activated protein kinase C receptor as well as that of aquaglyceroporin and mitogen-activated protein kinase.
The failure rate of the L-AmB treatment was 27.5%, which was higher than that reported in our previous paediatric series (17%) (13). Furthermore, the emergence of acquired drug resistance to L-AmB has been reported in Leishmania donovani (22) and Leishmania mexicana (23) isolates. Amphotericin-resistant lines showed marked differences in membrane sterol compositions, which probably reduced the ability of the drug to bind sterols as its main mode of action. It remains to be studied whether L. tropica strains in Israel develop drug resistance and the nature of the underlying mechanism. The significant treatment failure and adverse effect rates for both L-AmB and IV SSG in the current study emphasize the need for novel drug treatments for CL that are both effective and safe. Miltefosine is emerging as an attractive alternative as an oral medication that can be taken at home with good tolerance. The efficacy of miltefosine in paediatric patients with L. tropica CL has been shown recently (17, 24). In the current retrospective study, data about miltefosine treatment was not included.
Limitations
This study has several potential limitations. The main limitation is its retrospective nature and the lack of a placebo-control group. Furthermore, many patients were treated with several treatment modalities, making it difficult to assess the efficacy of various individual treatments and their long-term effects.
Conclusion
While the efficacy of both systemic treatments, L-AmB and IV SSG, was the same (70%), the former was required for a considerably shorter duration per protocol and had significantly fewer adverse effects. Thus, L-AmB is an efficacious and safe systemic treatment option for L. tropica CL, and the new treatment modality of miltefosine requires further evaluation.
The authors have no conflicts of interest to declare.
REFERENCES
- Alvar J, Velez ID, Bern C, Herrero M, Desjeux P, Cano J, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 2012; 7: e35671.
- Pigott DM, Golding N, Messina JP, Battle KE, Duda KA, Balard Y, et al. Global database of leishmaniasis occurrence locations, 1960–2012. Sci Data 2014; 1: 140036.
- Jaffe CL, Baneth G, Abdeen ZA, Schlein Y, Warburg A. Leishmaniasis in Israel and the Palestinian Authority. Trends Parasitol 2004; 20: 328–332.
- Schlein Y, Warburg A, Schnur LF, Le Blancq SM, Gunders AE. Leishmaniasis in Israel: reservoir hosts, sandfly vectors and leishmanial strains in the Negev, Central Arava and along the Dead Sea. Trans R Soc Trop Med Hyg 1984; 78: 480–484.
- Jacobson RL, Eisenberger CL, Svobodova M, Baneth G, Sztern J, Carvalho J, et al. Outbreak of cutaneous leishmaniasis in northern Israel. J Infect Dis 2003; 188: 1065–1073.
- Hepburn NC. Cutaneous leishmaniasis. Clin Exp Dermatol 2000; 25: 363–370.
- Klaus S, Frankenburg S. Cutaneous leishmaniasis in the Middle East. Clin Dermatol 1999; 17: 137–141; discussion 105–136.
- Momeni AZ, Aminjavaheri M. Treatment of recurrent cutaneous leishmaniasis. Int J Dermatol 1995; 34: 129–133.
- Moskowitz PF, Kurban AK. Treatment of cutaneous leishmaniasis: retrospectives and advances for the 21st century. Clin Dermatol 1999; 17: 305–315.
- Magill AJ, Grögl M, Gasser RA, Jr, Sun W, Oster CN. Visceral infection caused by leishmania tropica in veterans of Operation Desert Storm. N Engl J Med 1993; 328: 1383–1387.
- Aronson N, Herwaldt BL, Libman M, Pearson R, Lopez-Velez R, Weina P, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis 2016; 63: e202–e264.
- Heras-Mosteiro J, Monge-Maillo B, Pinart M, Lopez Pereira P, Reveiz L, Garcia-Carrasco E, et al. Interventions for Old World cutaneous leishmaniasis. Cochrane Database Syst Rev 2017; 12: CD005067.
- Solomon M, Schwartz E, Pavlotsky F, Sakka N, Barzilai A, Greenberger S. Leishmania tropica in children: a retrospective study. J Am Acad Dermatol 2014; 71: 271–277.
- Herwaldt BL, Berman JD. Recommendations for treating leishmaniasis with sodium stibogluconate (Pentostam) and review of pertinent clinical studies. Am J Trop Med Hyg 1992; 46: 296–306.
- Solomon M, Pavlotsky F, Leshem E, Ephros M, Trau H, Schwartz E. Liposomal amphotericin B treatment of cutaneous leishmaniasis due to Leishmania tropica. J Eur Acad Dermatol Venereol 2011; 25: 973–977.
- Wortmann G, Zapor M, Ressner R, Fraser S, Hartzell J, Pierson J, et al. Lipsosomal amphotericin B for treatment of cutaneous leishmaniasis. Am J Trop Med Hyg 2010; 83: 1028–1033.
- Kamink S, Masih B, Ali N, Ullah A, Khan SJ, Ashraf S, et al. Effectiveness of miltefosine in cutaneous leishmaniasis caused by Leishmania tropica in Pakistan after antimonial treatment failure or contraindications to first line therapy-A retrospective analysis. PLoS Negl Trop Dis 2021; 15: e0008988.
- Monroy-Ostria A, Nasereddin A, Monteon VM, Guzmán-Bracho C, Jaffe CL. ITS1 PCR-RFLP diagnosis and characterization of leishmania in clinical samples and strains from cases of human cutaneous leishmaniasis in States of the Mexican Southeast. Interdiscip Perspect Infect Dis 2014; 2014: 607287.
- Ponte-Sucre A, Gamarro F, Dujardin JC, Barrett MP, Lopez-Velez R, Garcia-Hernandez R, et al. Drug resistance and treatment failure in leishmaniasis: a 21st century challenge. PLoS Negl Trop Dis 2017; 11: e0006052.
- Hadighi R, Mohebali M, Boucher P, Hajjaran H, Khamesipour A, Ouellette M. Unresponsiveness to Glucantime treatment in Iranian cutaneous leishmaniasis due to drug-resistant Leishmania tropica parasites. PLoS Med 2006; 3: e162.
- Karamian M, Bojd MS, Salehabadi A, Hemmati M, Barati DA. Effectiveness of meglumine antimoniate against L. tropica in a recently emerged focus of cutaneous leishmaniasis in Birjand, eastern Islamic Republic of Iran. East Mediterr Health J 2015; 21: 280–286.
- Purkait B, Kumar A, Nandi N, Sardar AH, Das S, Kumar S, et al. Mechanism of amphotericin B resistance in clinical isolates of Leishmania donovani. Antimicrob Agents Chemother 2012; 56: 1031–1041.
- Mwenechanya R, Kovářová J, Dickens NJ, Mudaliar M, Herzyk P, Vincent IM, et al. Sterol 14α-demethylase mutation leads to amphotericin B resistance in Leishmania mexicana. PLoS Negl Trop Dis 2017; 11: e0005649.
- Ollech A, Solomon M, Horev A, Reiss-Huss S, Ben-Amitai D, Zvulunov A, et al. Cutaneous leishmaniasis treated with miltefosine: a case series of 10 paediatric patients. Acta Derm Venereol 2020; 100: adv00322.