Intractable itch is defined as a chronic itchy state in which the cause cannot be removed or otherwise treated through the general course of medical practice (1). Many of these conditions include dermatological aetiologies (2, 3), in addition to other systemic aetiologies. Quality of life, pertaining to sleep, depression, anxiety, and relationships, has been found to be significantly impaired in patients with severe itch (4).
There is data to suggest that in chronic itch of multiple types there is an imbalance of mu opioid receptor overactivation and kappa opioid receptor downregulation (5). Therefore, the use of butorphanol, a kappa-opioid receptor agonist and mu-opioid receptor antagonist to decrease itch.
There are a few case series reporting on the use of intranasal butorphanol as treatment for intractable itch with results finding butorphanol to be highly effective at a rapid speed of onset, and well tolerated, for different itch-associated chronic etiologies (5, 6). However, the current literature lacks any larger scale study on the effectiveness of butorphanol over a longer period.
MATERIALS AND METHODS
Patients who were prescribed intranasal butorphanol for the treatment of chronic pruritus were identified and evaluated for this study using the following parameters: date of medication prescription from November 2017 to December 2021 at the University of Miami Hospital and satellite clinics, and patient age at prescription date ≥ 18 years. Patients were instructed to administer at least 1 puff of 1 mg equivalent, and up to 4 mg equivalent (4 puffs) interspaced as a divided dose throughout the day, of intranasal butorphanol 10 mg/ml. Patients used intranasal butorphanol every day between the first and second visit, unless otherwise indicated. Itch was quantified using a pruritus numerical rating score (NRS) at the initial visit prior to intranasal butorphanol initiation and at the subsequent visit(s). Pruritus NRS is a validated tool that is reliable and sensitive in assessing itch in patients with atopic dermatitis (AD) (7); it is embedded within the University of Miami electronic medical record system. Important information was extracted in a comprehensive chart review. Patient data were de-identified and statistically analysed using a paired t-test and a 2-tailed Wilcoxon signed-rank test. Statistical significance was assigned at p < 0.05.
RESULTS
A total of 33 adults were analysed in this retrospective chart review. Butorphanol was prescribed for a variety of pruritic diseases. The mean ± standard deviation (SD) length of itch history prior to butorphanol initiation was approximately 43 ± 63.8 months (Table I).
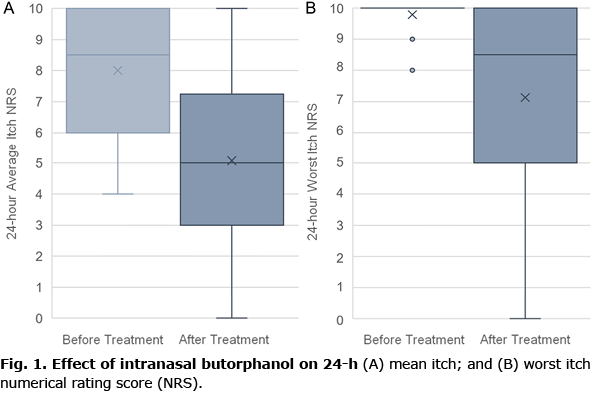
Of the total 33 patients, 26 (79%) patients were re-evaluated following treatment, while 7 (21%) patients were lost to follow-up (3; 9%) or never began treatment (4; 12%). The remainder of the results will evaluate only the participants who were enrolled in this study, received butorphanol treatment, and were reassessed in a follow-up visit. Two participants were excluded from 24-h worst itch NRS statistical analyses due to lack of obtained data in either pre- or post-assessments. The average-itch NRS mean was 8.00 ± 2.21 and the worst-itch NRS mean was 9.79 ± 0.51 for the 26 patients who were analysed within the last 5 years. Post-treatment, the average-itch NRS mean and the worst-itch NRS mean in this group were 5.08 ± 2.69 and 7.13 ± 3.39, respectively, at the second patient visit (Fig. 1A). The average period between the first and second visits was approximately 2.6 ± 3.35 months; although, many patients reported that the efficacy of treatment occurred following the first few days. The results demonstrate significant reduction in average- and worst-itch NRS (p < 0.002). Fourteen (54%) patients experienced a 4-point or more decrease in average-itch NRS and 7 (29%) patients experienced a 4-point or more decrease in worst-itch NRS. Three (12%) patients, who otherwise did not demonstrate meaningful reduction in itch NRS, reported anecdotal improvement in itch. In patients who experienced either a quantifiable or qualifiable improvement in pruritus, the antipruritic effect of the drug was noted to occur after 1 use in the majority of cases. Amongst these 26 participants, 20 (77%) patients continued treatment with intranasal butorphanol for the intended treatment period (until subsequent follow-up visit), while 6 (23%) patients discontinued treatment prior to the intended treatment period due to adverse effects and/or intolerance.
Nine (35%) patients reported at least 1 side-effect. Five of the 7 patients discontinued treatment early due to adverse effects and 1 patient who experienced improved itch with butorphanol eventually stopped treatment because of intolerance to side-effects. The most common adverse effects include dizziness and/or nausea (3 patients; 12%), abnormal dreams (2 patients; 8%), feelings of intoxication or altered sense of consciousness (2 patients; 8%) and drowsiness (2 patients; 8%).
DISCUSSION
Itch can result from an imbalance between itch-promoting mu-opioid receptors and itch-diminishing kappa-opioid receptors; altering the opioid axis can therefore decrease the perception of pruritus (8). Thus far, nalbuphine, nalfurafine, and difelikefalin are emerging opioid receptor agonist/antagonists that have antipruritic properties (9). Nalbuphine is a mu-opioid antagonist and kappa-opioid agonist that is approved for treatment of pain, but has been studied for the treatment of pruritus (10–12). Kappa opioid agonists, nalfurafine and difelikefalin, have demonstrated efficacy in reducing itch, with the former approved only in Japan for uraemic itch and CKD-associated pruritus, and the latter in the USA and Europe for CKD-associated pruritus in the setting of haemodialysis; however, difelikefalin has not yet launched in the market (8, 13, 14). Currently, there are numerous clinical trials in the USA investigating the efficacy of these treatments in itchy conditions. As butorphanol is available in the US market, it could be used off-label for the treatment of chronic itch.
The results of this study reveal that butorphanol is efficacious in reducing the perception of itch at a rapid rate for many different itch-associated aetiologies, and is well-tolerated, corroborating results from prior studies (5, 6). A total of 17 (65%) patients had either numerical or subjective evidence of significant pruritic improvement by the end of the study (Fig. 1B).
The itchy condition that responded most frequently to treatment with butorphanol was chronic pruritus of unknown origin (9; 35%). The next most frequent responses were neuropathic itch (5; 19%) and chronic kidney disease-associated pruritus (3; 12%).
Most adverse effects involved the central nervous system. Frequently, these side-effects occurred after first use or early in the treatment course. These effects are similar to those reported with other kappa opioid agonists in clinical trials (14, 15). The mixed properties of agonism and antagonism comprising butorphanol may lend itself to minimal abuse potential, despite its classification as a schedule IV-controlled substance. None of the patients in this study reported abuse of treatment, leading us to believe the addictive potential is low. Interestingly, the mechanism of action of nalbuphine is the same as butorphanol, yet it is not categorized as a controlled substance.
In conclusion, intranasal butorphanol may be a rapidly effective choice of treatment for refractory, intractable chronic pruritus of different aetiologies. Limitations of this study include the retrospective study design, in which the treatment was not evaluated in a double-blind, placebo-controlled manner, and thus there was variability in treatment protocol. Further study of treatment with butorphanol for severe, long-term itch should be investigated in a large-scale, controlled trial.
Conflicts of interest: GY has been an investigator and consultant to Galderma, Pfizer, Sanofi Regeneron, Eli Lilly, Bellus, Kiniksa, Leo and Trevi. The other authors have no conflicts of interest to declare.
REFERENCES
- Yosipovitch G, Greaves MW, McGlone F. Itch: basic mechanisms and therapy. Boca Raton, CRC Press; 2004.
- Umehara Y, Kiatsurayanon C, Trujillo-Paez JV, Chieosilapatham P, Peng G, Yue H, et al. Intractable itch in atopic dermatitis: causes and treatments. Biomedicines 2021; 9: 229.
- Zeidler C, Yosipovitch G, Ständer S. Prurigo nodularis and its management. Dermatol Clin 2018; 36: 189–197.
- Zachariae R, Lei U, Haedersdal M, Zachariae C. Itch severity and quality of life in patients with pruritus: preliminary validity of a Danish adaptation of the itch severity scale. Acta Derm Venereol 2012; 92: 508–514.
- Dawn AG, Yosipovitch G. Butorphanol for treatment of intractable pruritus. J Am Acad Dermatol 2006; 54: 527–531.
- Khanna R, Kwon CD, Patel SP, Belzberg M, Williams KA, Khanna R, et al. Intranasal butorphanol rescue therapy for the treatment of intractable pruritus: a case series from the Johns Hopkins Itch Clinic. J Am Acad Dermatol 2020; 83: 1529–1533.
- Yosipovitch G, Reaney M, Mastey V, Eckert L, Abbé A, Nelson L, et al. Peak pruritus numerical rating scale: psychometric validation and responder definition for assessing itch in moderate-to-severe atopic dermatitis. Br J Dermatol 2019; 181: 761–769.
- Beck TC, Hapstack MA, Beck KR, Dix TA. Therapeutic potential of kappa opioid agonists. Pharmaceuticals (Basel) 2019; 12: 95.
- Reszke R, Krajewski P, Szepietowski JC. Emerging therapeutic options for chronic pruritus. Am J Clin Dermatol 2020; 21: 601–618.
- Mathur VS, Kumar J, Crawford PW, Hait H, Sciascia T. A multicenter, randomized, double-blind, placebo-controlled trial of nalbuphine ER tablets for uremic pruritus. Am J Nephrol 2017; 46: 450–458.
- Hawi A, Alcorn H, Jr, Berg J, Hines C, Hait H, Sciascia T. Pharmacokinetics of nalbuphine hydrochloride extended release tablets in hemodialysis patients with exploratory effect on pruritus. BMC Nephrol 2015; 16: 47.
- Weisshaar E, Szepietowski JC, Bernhard JD, Hait H, Legat FJ, Nattkemper L, et al. Efficacy and safety of oral nalbuphine extended release in prurigo nodularis: results of a phase 2 randomized controlled trial with an open-label extension phase. J Eur Acad Dermatol Venereol 2022; 36: 453–461.
- Kremer AE. What are new treatment concepts in systemic itch? Exp Dermatol 2019; 28: 1485–1492.
- Deeks ED. Difelikefalin: first approval. Drugs 2021; 81: 1937–1944.
- Kumagai H, Ebata T, Takamori K, Miyasato K, Muramatsu T, Nakamoto H, et al. Efficacy and safety of a novel ĸ-agonist for managing intractable pruritus in dialysis patients. Am J Nephrol 2012; 36: 175–183.