Patients with psoriasis are at risk of developing psoriatic arthritis, which can lead to irreversible joint damage. However, a proportion of patients with psoriasis and concomitant psoriatic arthritis remain undiscovered in practice. The aims of this study were: to prospectively determine prevalence, characteristics, and disease burden of psoriatic arthritis in a psoriasis population; and to determine the prevalence and characteristics of patients with active psoriatic arthritis, who were not under rheumatological care. Patients with psoriasis were screened by a rheumatologist at the dermatology outpatient clinic for psoriatic arthritis. Patients with suspected active psoriatic arthritis who were not seeing a rheumatologist were referred to a rheumatologist for confirmation. The total prevalence of psoriatic arthritis in this observational, prospective cohort (n = 303) was 24%. Patients with psoriasis with concomitant psoriatic arthritis had longer duration of skin disease and more often a treatment history with systemic therapies. In this academic, specialized, setting, 2.3% of patients (n = 7), were not receiving rheumatological care despite having active psoriatic arthritis. These patients were characterized by a combination of low (perceived) disease burden and low yield of screening questionnaires, making it difficult for dermatologists to discover psoriatic arthritis in these patients. Thus, screening for more subtle active arthritis in patients with psoriasis in a dermatology setting could be improved.
Key words: psoriasis; psoriatic arthritis; screening.
Accepted Jul 12, 2022; Epub ahead of print Jul 12, 2022
Acta Derm Venereol 2022; 102: adv00768.
DOI: 10.2340/actadv.v102.2225
Corr: Tamara W. van Hal, Department of Rheumatology, Sint Maartenskliniek, Hengstdal 3, PO Box 9011, NL-6500 GM Nijmegen, The Netherlands. E-mail: t.vanhal@maartenskliniek.nl
SIGNIFICANCE
Patients with psoriasis are at risk of psoriatic arthritis. However, it is difficult for dermatologists to interpret joint complaints. In this study, 300 patients with psoriasis visiting the dermatology outpatient clinic were screened by a rheumatologist, revealing that 1 in 4 patients in this cohort had psoriatic arthritis. Patients with arthritis had a longer disease duration of psoriasis, more joint pain, and and more often had osteoarthritis. Patients with active arthritis not treated by a rheumatologist had mild complaints. The sometimes subtle signs of psoriatic arthritis and its high prevalence underline the need for better screening tools to aid dermatologists.
INTRODUCTION
Psoriatic arthritis (PsA) is a debilitating immune- mediated inflammatory disease of joints and entheses, which can lead to permanent joint damage (1). Adequate and early treatment of PsA improves joint function and quality of life (QoL) (2). Therefore, it is crucial to discover and treat patients with PsA as soon as possible. The population most at risk of PsA are patients with psoriasis (PsO): 1 in 3 patients with PsO will develop PsA (3). Because PsO usually presents itself before the onset of PsA, dermatologists are in a unique position to screen patients with PsO for the presence of PsA (4).
Unfortunately, in patients with PsO at the dermatology clinic, PsA is frequently undiscovered (5). While this leads to undertreatment of joint complaints in the individual patients, it also leads to an underestimation of the prevalence of PsA in the PsO population. This is exemplified by a lower prevalence of PsA in PsO in population studies (where PsA was scored by analysing registered diagnoses in electronic health files) compared with observational studies (where PsA was actively sought in patients with PsO) (6). To aid dermatologists in discovering patients with PsA, several screening questionnaires have been developed (7–11). However, when tested in external validation cohorts, the sensitivity of these questionnaires differed widely, ranging from 24% to 92% (12). This means that, even with the use of these validated questionnaires, patients with PsA elude detection. Also, the predictive performance of the screening questionnaires is known to be worse in patients who have undiscovered PsA compared with patients with known PsA (13, 14).
In designing the screening questionnaires, studies have been hampered by a low number of patients with PsO with newly discovered PsA (7, 8). To improve power, some groups have chosen to increase the group of PsA cases by adding patients with already known PsA from the rheumatology department (7, 9). However, patients with undiscovered PsA may differ from those who are already known and treated at the rheumatology department, which may lead to underperformance of the screening tools in this specific population. It is therefore important to increase our knowledge of the population of PsO patients with PsA, especially with regard to those who are not actively treated by a rheumatologist.
The aim of the DAPPER study (Discovery of Arthritis in Psoriasis Patients for Early Rheumatological Referral) was to identify and describe patients with PsO and concomitant PsA at the dermatology outpatient clinic. Firstly, the current study determined the prevalence, characteristics, and disease burden of PsA in a PsO population. Furthermore, the study investigated the prevalence of patients with active PsA, who were not (yet) under current rheumatological care. We further characterized the medical history and joint complaints of these patients with active PsA without current rheumatological care. Finally, we examined whether the treatment, disease activity, or QoL of these patients with active PsA without rheumatological care changed after referral to a rheumatologist.
MATERIALS AND METHODS
Study setting and design
DAPPER is a prospective observational study, conducted at the department of dermatology of the Radboud University Medical Center (Radboudumc) from 1 June 2019 to 17 February 2022 (recruitment and data collection June 2019–June 2021, follow-up until February 2022 for newly discovered PsA patients). The Radboudumc is a national expertise centre for psoriasis. In line with this specialized setting, patients in certain study cohorts (e.g. patients using biologicals) are screened annually using the Psoriasis Epidemiology Screening Tool (PEST) questionnaire (7). However, patients outside these study cohorts are not routinely screened for the presence of PsA. The study protocol of the DAPPER study has been published in detail elsewhere (15). It was approved by the ethics committee of the region Arnhem-Nijmegen, Radboudumc (NL68137.091.18), and registered in the Dutch Trial Register (NTR 7604). The study was performed in accordance with the Declaration of Helsinki and Good Clinical Practice.
Participants
Patients with physician-diagnosed PsO, aged ≥ 18 years, currently being treated by a dermatologist, were eligible for inclusion. Patients were stratified 1:1:1 for current treatment (topical treatments only, conventional systemics, biologicals/small molecule inhibitors (biol/SMI)) to enable outcome assessment per treatment group. Current treatment may serve as a proxy for disease severity (16). A concomitant diagnosis of PsA was not an exclusion criterion. All patients gave written informed consent before inclusion in the study.
Study procedure
After informed consent, a study visit was planned adjacent to a regular outpatient visit with the dermatologist. During the study visit, patients were screened for suspicion of active PsA by a trained rheumatologist using a structured interview and physical examination. For the full list of parameters, see Appendix S1.
When there was a clinical suspicion of active PsA at the study visit, and the patient was not under current rheumatological care, the patient was referred to a rheumatologist. There, additional examinations were performed for confirmation or rejection of diagnosis (i.e. laboratory tests, and/or imaging such as ultrasound, X-ray, or magnetic resonance imaging (MRI)). When there was a clinical suspicion of active PsA, and the patient was already under current rheumatological care, the patient was advised to contact their treating rheumatologist. Current rheumatological care was defined as patients who were still actively visiting a rheumatologist for their PsA care, i.e. who had a planned appointment with their rheumatologist in the following year.
Patients with a rheumatologist-confirmed active PsA after referral were followed for 1 year. After 1 year, data on changes in treatment, PsA disease activity, and QoL were collected.
Outcomes
The primary outcome was the prevalence of concomitant PsA in patients with PsO. A patient was considered to have PsA if either he/she had received a previous diagnosis from a rheumatologist, or if he/she had a confirmed diagnosis of PsA after referral in this study. Active PsA was defined as having PsA, and at least 1 inflamed enthesis or joint (axial or peripheral) at the time of study visit. For axial arthritis or enthesitis, imaging was required to confirm active inflammation.
Groups were defined as either “PsO” (cutaneous PsO only) or “PsoPsA” (PsO with concomitant PsA). Demographic data and disease characteristics of PsO and PsoPsA were compared. Secondary outcome was the prevalence of active PsA not under the care of a rheumatologist in patients with PsO. Of these patients with PsoPsA, medical history and joint complaints were described. Also, changes in treatment, disease activity, and QoL 1 year after referral of these patients were assessed by comparing scores on the Psoriatic Arthritis Disease Activity Score (PASDAS), Dermatology Life Quality Index (DLQI), and Psoriatic Arthritis Impact of Disease (PsAID) with measurements at the time of referral (17–19).
Statistical analysis
Continuous data were described with means (standard deviation; SD) or medians (interquartile ranges; IQR), when appropriate. Categorical data were described as absolute frequencies with percentages.
Prevalence estimates were calculated as n per 100 patients with PsO, with 95% confidence intervals (95% CI). Patients with unclear diagnoses were classified as not having PsA, but a sensitivity analysis was performed in which patients with unclear diagnosis were classified as cases.
Differences between groups were tested with unpaired Student’s t-test or Mann–Whitney U (continuous data), or χ2/Fisher’s exact test (categorical data) when appropriate. Missing data were not imputed. Patients with suspected PsA after study visit, who were unable or unwilling to visit a rheumatologist for confirmation of diagnosis, were defined as “unclear diagnosis”. Patients with unclear diagnoses were not included in the comparisons between PsO and PsoPsA groups.
Bonferroni correction for multiple testing was applied, with an alpha of 0.001 (0.05/58 tests) being considered significant. Data were analysed using SPSS Statistics software, version 25 (IBM, Armonk, NY, USA).
RESULTS
Participants
Fig. 1A shows the flow chart of included patients. A total of 516 patients (consecutive per treatment group) were approached, of whom 304 agreed to participate. Patients used topical treatments only (n = 101), conventional systemics (n = 102), or biol/SMI (n = 101). One patient dropped out during the study visit because of inability to undergo physical examination. Four patients had a clinical suspicion of PsA during the study visit, but refrained from visiting a rheumatologist (n = 3 declined referral, n = 1 intercurrent illness). Table I shows the characteristics of the included patients. Mean age at inclusion was 54 years; 36% of patients were female (109/304).

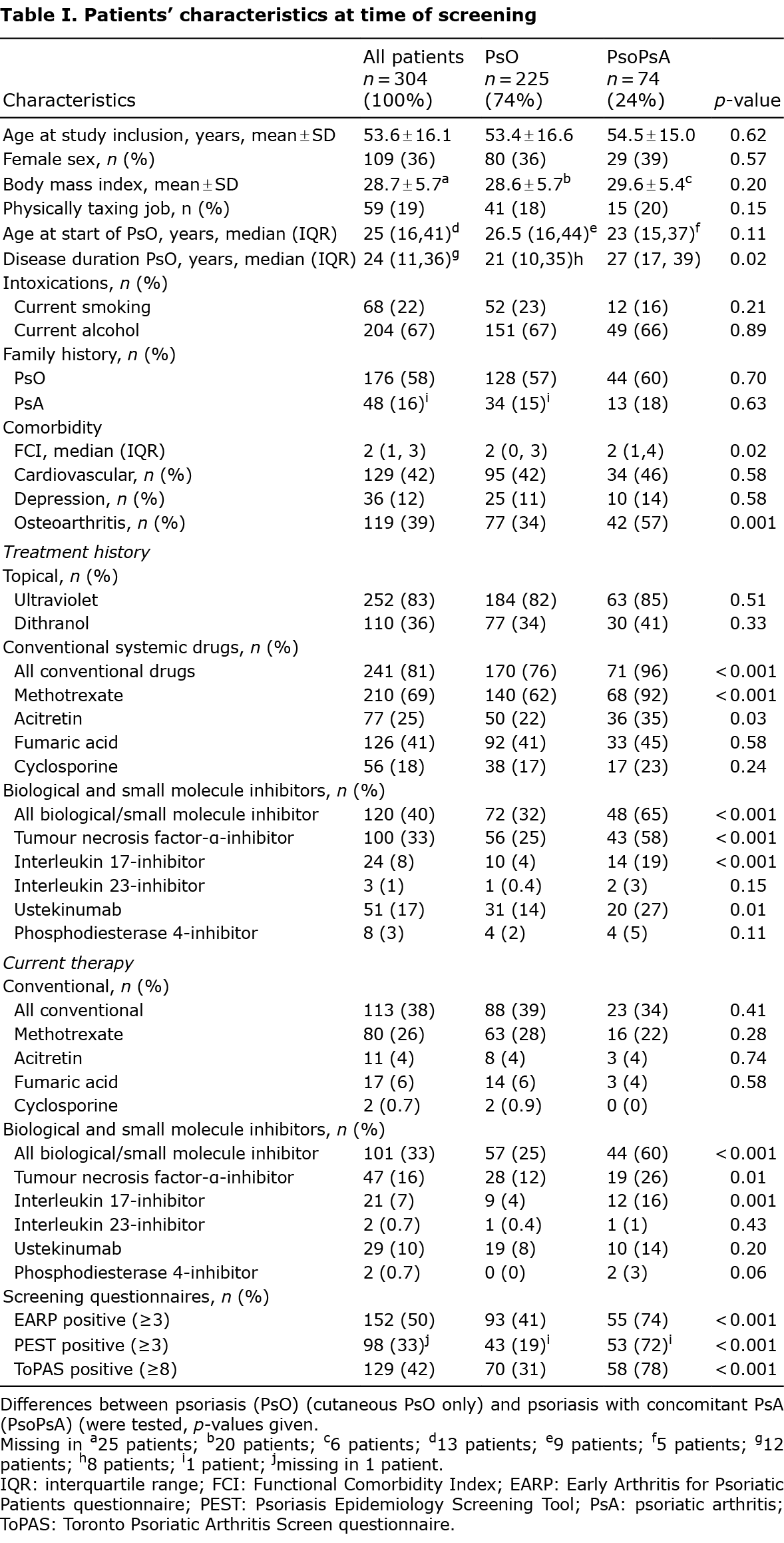
Prevalence of psoriatic arthritis
Fig. 1B shows the diagnosis of all patients. After excluding the patients in whom no diagnosis could be made (n = 5: 1 unfulfilled screening, 4 unfulfilled referral), the prevalence of PsA in this treatment-stratified cohort was 24.4% (74/304; 95% CI 21.9–26.8%). The prevalence of PsA was 11.9% (12/101; 95% CI 8.7–15.1%) in the topicals only group, 17.5% (18/103; 95% CI 13.7–21.2%) in the conventional systemics group, and 44.0% (44/100; 95% CI 39.0–49.0%) in the biol/SMI group. A sensitivity analysis, where all patients with an unclear diagnosis were classified as cases, showed similar results (total prevalence 25.7%, 95% CI 23.2–28.2%; topicals only 14.9%; 95% CI 12.2–19.5%; conventional systemics 18.4, 95% CI 14.6–22.2%; biologicals unaltered).
Characteristics and disease burden of patients with psoriasis and psoriasis/psoriatic arthritis
Tables I and II show the characteristics and disease burden of the cohort. When applying Bonferroni correction, patients with PsO differed from patients with PsoPsA with regard to: a previous diagnosis of osteoarthritis (PsO 77/225, 34%; PsoPsA 42/74, 57%; p = 0.001), ever use of conventional systemics (PsO 170/224, 76%; PsoPsA 71/74, 96%; p < 0.001), ever use of biol/SMI (PsO 72/225, 32%; PsoPsA 48/74, 65%; p < 0.001), current use of biol/SMI (PsO 57/225, 25%; PsoPsA 44/74, 60%; p < 0.001), patient-reported joint pain in proximal joints (PsO 92/225, 41%; PsoPsA 53/75, 72%; p < 0.001), and number of swollen joints at physical examination (p < 0.001). When applying an explorative cut-off of p < 0.05, the study also found differences in PsO skin disease duration (PsO 21 years (10, 35); PsoPsA 27 years (17, 39); p = 0.02 (median, IQR)), current joint pain (PsO 159/225, 71%; PsoPsA 63/74, 85%; p = 0.01), morning stiffness with a duration of more than 30 min (PsO 26/225, 12%; PsoPsA 19/74, 26%; p = 0.003) and number of tender joints at physical examination (p = 0.02). Sensitivity of used screening questionnaires was 74%, 72%, and 78% for EARP, PEST, and ToPAS, respectively.
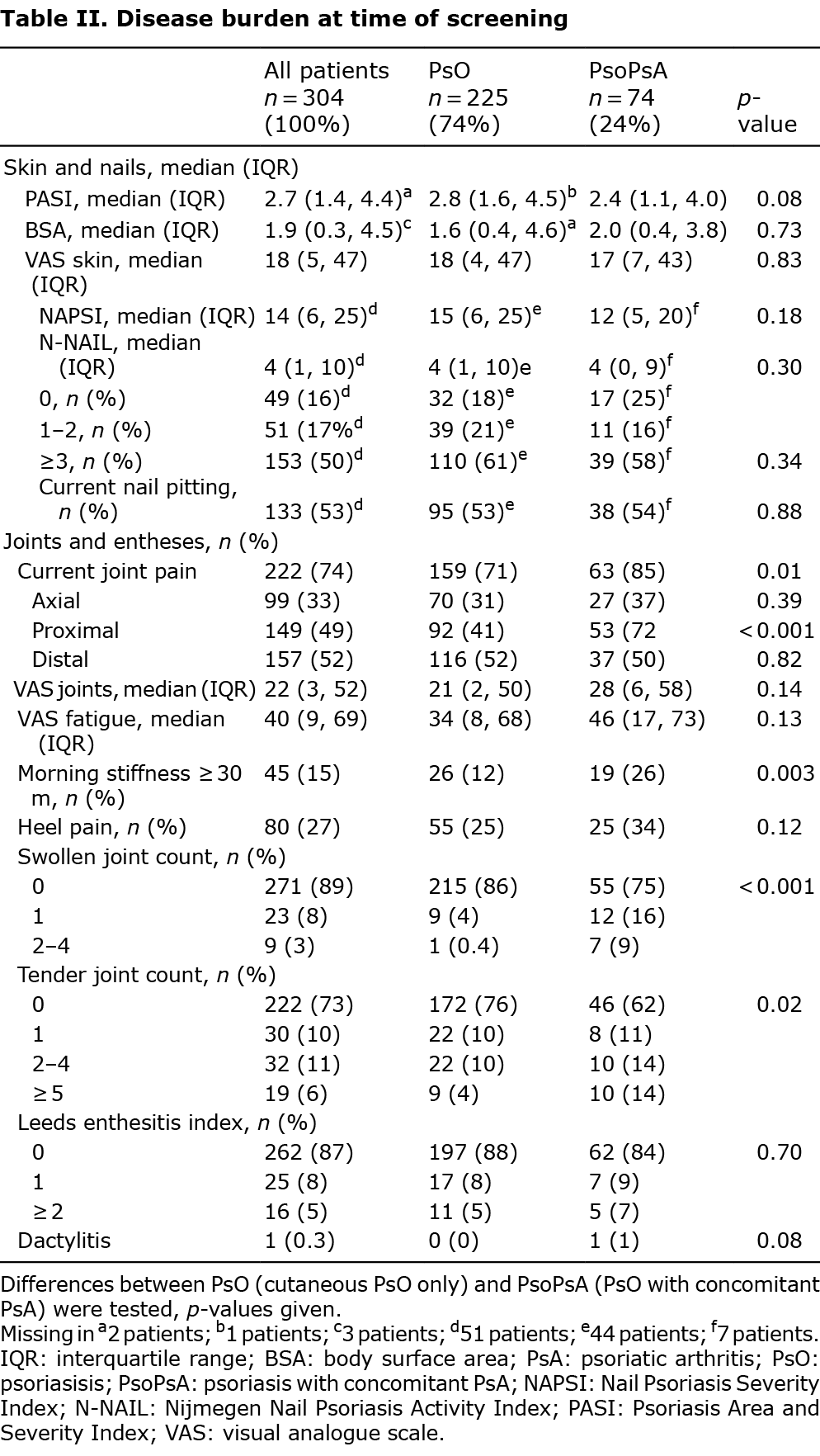
Suspicion of active psoriatic arthritis, in patients not under rheumatological care
Table III shows the characteristics of the patients who were referred to the department of rheumatology (n = 26 suspected of active PsA, of which n = 22 referred). In 9/22 patients with suspicion of active PsA not under rheumatological care, the diagnosis PsA was confirmed. In 7 out of these 9 patients the PsA was deemed active (32% of all referred patients), which accounted for 2.3% of the entire cohort. Of these patients, 5/7 did not have the diagnosis previously; 2/7 were previously diagnosed with PsA, but were not currently treated by a rheumatologist. In 2/9 patients additional imaging did not reveal active musculoskeletal inflammation at the time of their visit to the rheumatology department. These 2 patients, who were in remission for PsA, both had a previous diagnosis of PsA but were not under the current care of a rheumatologist.
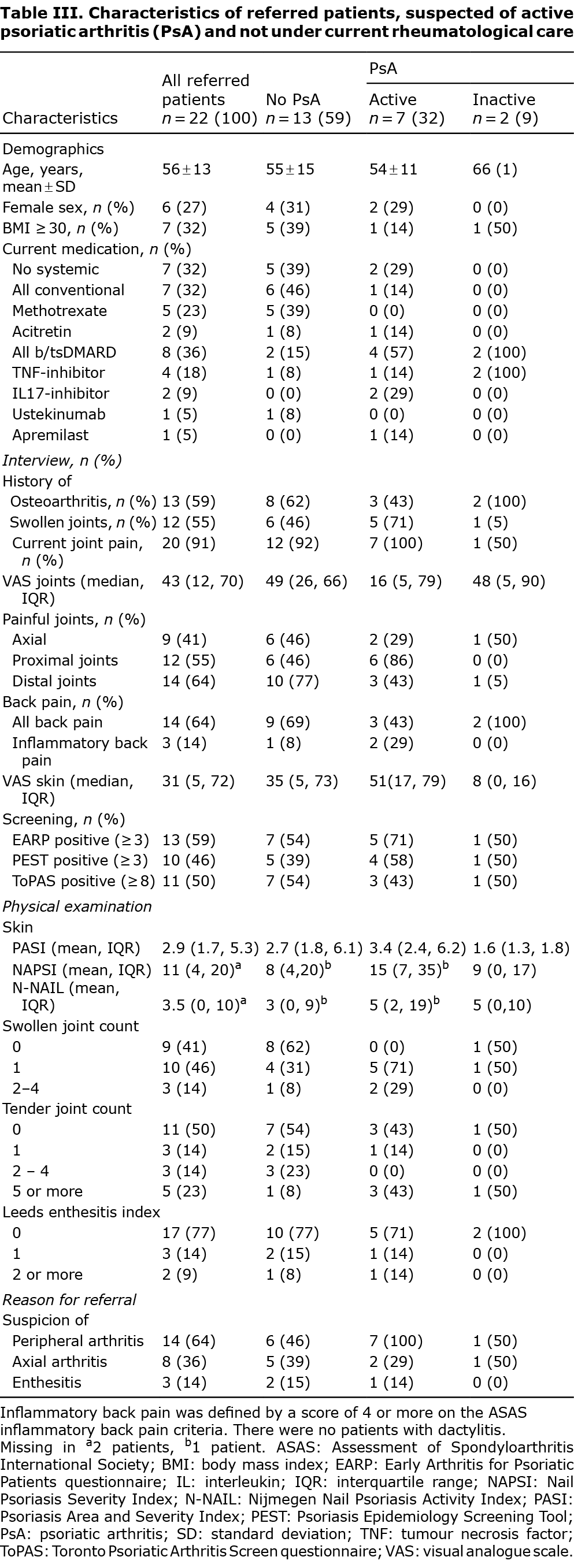
Baseline characteristics of patients with confirmed active psoriatic arthritis upon new referral to the rheumatology clinic
Table IV and Table SI show the characteristics of the 7 patients with confirmed active PsA, who were not under rheumatological care. All patients (7/7) fulfilled the Classification Criteria for Psoriatic Arthritis (CASPAR); 2/7 patients showed irreversible joint changes (i.e. erosions) on imaging. Five out of 7 patients presented in the study visit with a mono-arthritis. Only 2/7 patients indicated a significant burden of joint pain (VAS joints ≥ 50 mm) and impact on QoL (PsAID12 ≥ 4.0) at the study visit. All patients with complete clinical data (6/6) were in moderate disease activity according to PASDAS (range: 3.8–5.3). The screening questionnaires identified 2/5 patients with a new diagnosis, and 2/2 patients with previously known PsA.
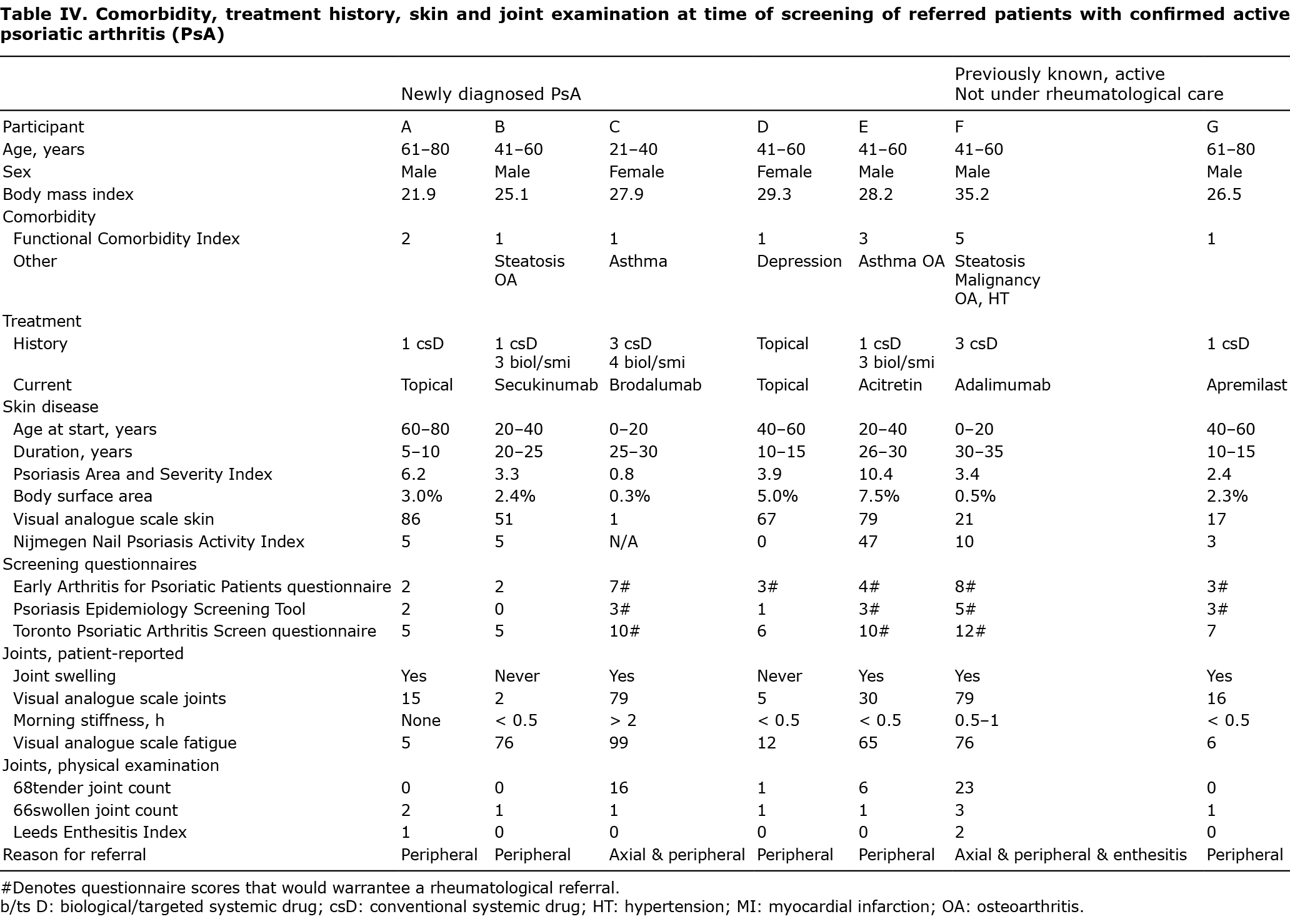
Longitudinal follow-up of patients with confirmed active psoriatic arthritis upon new referral to the rheumatologist
Table SI shows the follow-up data of the 7 referred patients with confirmed active PsA. In 6/7 patients, rheumatological referral led to 1 or more treatment changes (intra-articular injections n = 3, start conventional systemics n = 3, switch in biol/SMI n = 1; 1 patient started conventional systemic after intra-articular injections). In 1/7 patients, treatment was changed by the dermatologist already from a conventional systemic drug to a biological. During follow-up, 1/7 patients stopped all systemic medications after a COVID-19 infection, and refused further systemic rheumatological or dermatological follow-up. Regarding disease activity, 5/6 patients showed improvement in the number of swollen joints after 1 year. Two out of 4 patients with complete PASDAS follow-up were in low disease activity (PASDAS ≤ 3.2). Regarding health-related quality of life (HR-QoL), before referral, 4/7 patients showed a large burden of PsO/PsA on their QoL, as measured by DLQI or PsAID12 (DLQI ≥ 5 or PsAID12 ≥ 4, respectively). After 1 year, 3/7 patients showed a large burden of PsA (PsAID12 ≥ 4). Of these 3 patients, 2 still had active PsA despite treatment changes (PASDAS ≥ 5.4), while the other patient reported a large burden of skin disease (DLQI ≥ 5).
DISCUSSION
This prospective observational study identified patients with PsO and concomitant PsA in the dermatology outpatient clinic via a structured interview and physical examination by a trained rheumatologist. A prevalence of PsA in PsO of 24% was found in the entire cohort. When separated by current treatment modality, the prevalence of PsA in PsO was 12% for topicals only, 18% for conventional systemics, and 44% for biol/SMI. When comparing PsoPsA with PsO patients, patients with PsoPsA were more often diagnosed with osteoarthritis, had a higher functional comorbidity index, had more often used conventional systemic medication and biologics, had a longer duration of skin disease, and more often reported joint pain and morning stiffness. Using extensive screening, this study identified 7 (2.3%) patients with PsO with active PsA who were not under current rheumatological care. These patients were referred to the rheumatologist: conventional systemic therapy was started in 3/7, biologic therapy was switched in 1/7 patients, local glucocorticoid joint injections were given to 3/7 patients. After 1 year, 5/6 patients showed improvement in arthritis.
One in 4 patients in the current PsO cohort had concomitant PsA. These results are in line with those of the systematic review of Alinaghi et al, who found a pooled prevalence of 22.7% (95 CI 20.6–25.0%) for PsA in PsO patients in Europe (6). The increase in PsA prevalence parallel to an increase in treatment intensity is also comparable to previous studies (3, 20). A possible explanation for this phenomenon could be that the increase in treatment severity represents an increase in skin disease severity. For instance, Ogdie et al. showed that a higher affected BSA is associated with a higher incidence of PsA (16).
Characteristics differed between PsO and PsoPsA patients. It is known that PsO precedes PsA in the majority of patients. The PsoPsA group showed a longer disease duration compared with the PsO group, but their current age did not differ. Indeed, the age at start of PsO showed a numerical difference, indicating that PsO was diagnosed at an earlier age in the PsoPsA group. Patients with PsoPsA were more often diagnosed with osteoarthritis, which could be detection bias, due to the fact that they visited a rheumatologist more often, or misclassification where PsA symptoms were interpreted as osteoarthritis. Although the patients more often used conventional systemic medication and biologics, a proportion of the newly detected patients were on conventional systemic or biologic treatment, which is in line with previous studies (3, 21, 22).
Of interest, in the current study cohort, one-third of the patients (28/79, both known and unknown PsA patients) still had active PsA when screened. However, the current cohort contained only 7 patients with active PsA not under current rheumatological care, of whom 5 were undiagnosed. This proportion is lower than the 15.5% undiagnosed cases reported in the meta-analysis of Villani et al. (5). The setting and cohort composition might contribute to these differences. The current study cohort consisted of 3 treatment groups (topical, conventional systemic and biol/SMI) and the setting was a PsO expertise centre in which patients on biologics were already screened on a regular basis using the PEST questionnaire. In this specialized academic setting, dermatologists could have had more time during their consultations to ask for joint complaints, compared with dermatologists working in other settings. Because, ideally, all active cases of PsA are discovered and treated, this relatively low number of patients with newly discovered PsA in this cohort may be a hopeful sign that improved detection is feasible.
Regarding these 7 active PsA patients not under rheumatological care, 3 further factors are worth mentioning. First, in these patients, the disease burden of PsA was relatively low: 5/7 patients presented with a mono-arthritis, and patients did not report a significant burden of joint pain, or a significant impact of PsA complaints on their HR-QoL. Secondly, 2/7 patients were already known to have PsA, but were not under treatment of a rheumatologist anymore. Thirdly, the yield of the screening PsA questionnaires (e.g. PEST) in these patients was low: only 2/5 previously undiscovered PsA patients would have been marked as being suspect for PsA. Previous research also showed a lower sensitivity of the screening questionnaires in patients without a previous PsA diagnosis (14). This can be partially explained by the fact that both PEST and ToPAS ask whether a patient has been diagnosed with arthritis previously, providing all patients with previously diagnosed PsA with an extra point (7, 9).
One of the aims of the current research was to describe the changes in treatment, disease activity and QoL in the patients with active PsA who were referred to the rheumatologist. While the arthritis improved in the majority of the patients, it is notable that that 3/7 patients still experience a significant burden of PsA 1 year after referral. In 2/7 patients, this can be explained by the fact that there was still a high disease activity of PsA, as reflected by PASDAS. Unfortunately, studies have shown that, in clinical practice, a significant proportion of patients with PsA still have active disease, despite treatment (23, 24). Even in the stringent treat-to-target TICOPA trial, only 62% of patients undergoing protocolized tight control showed a significant response in joint scores (ACR20) (2). Thus, evaluation of the effect of PsA screening and referral on the disease burden experienced by patients is a valuable addition to the PsO/PsA research agenda.
Study strengths and limitations
The strengths of this study are the thorough interview and physical examination of all patients by a trained rheumatologist, and the setting in the dermatology outpatient clinic. Instead of using questionnaires with known low sensitivity, the study employed a rheumatologist to assess all patients (13, 14). As rheumatologist diagnosis is the gold standard, the risk of misclassification using this process was deemed very low (25). By placing this rheumatologist at the location of dermatological care, we ensured maximal participation of the patients with PsO. Thereby, we avoided “healthy participant” bias, where patients who are more interested in a healthy life(style) are more prone to join a study, as much as possible.
The limitations of this study are the setting in a tertiary hospital with special expertise in PsO care. This hampers the translation to non-academic cohorts, thereby abating the external validity. When comparing the current academic cohort with a nationwide cohort of patients approached via the Dutch Psoriasis Association, the current cohort is more often treated with systemic medication (conventional systemic 38% vs 26%, biologicals/SMI 33% vs 16%) and has a lower burden of skin disease (PASI 5.5 vs 2.7) (26). Moreover, a proportion of the patients in the current study cohort has already been screened regularly for PsA in the past. The treatment guideline of the Dutch Society for Dermatology and Venereology recommends alertness for the signs of PsA, the use of screening questionnaires is not formally recommended (27). As a consequence of the increased use of systemic medication and increased use of screening compared with non-academic dermatology clinics, our academic cohort showed a low amount of previously undetected PsA patients, making it difficult to determine the characteristics of these patients to aid detection in another setting.
Conclusion
The observational, prospective DAPPER study revealed that the prevalence of PsA in this tertiary centre was 24%, comparable to that in published literature. The patients with PsoPsA were characterized by a longer disease duration of PsO and a different treatment history with more conventional systemic and biologic therapies compared with patients with PsO. In this academic, specialized setting, where patients are already screened with questionnaires, many PsA cases were already identified. While this yield was already higher than in literature (5), still an additional 2.3% of patients were identified with active PsA who were not receiving rheumatological care. These patients were characterized by a combination of low (perceived) disease burden and low yield when using screening questionnaires, making it difficult for the dermatologist to discover PsA in these patients. While the current results show that it is possible to identify the majority of patients with PsA in regular care, improving current screening strategies for PsA in PsO is needed in order to detect more subtle active arthritis in patients with PsO in a dermatology setting.
ACKNOWLEDGEMENTS
The authors would like to thank the staff of the dermatology outpatient clinic of the Radboudumc (nurses, administration, photographers) for their support during this study.
Conflicts of interest: TvH received personal fees from Eli Lily and Novartis, non-financial support from UCB, outside the submitted work. MM received non-financial support from UCB, outside the submitted work. JvdR carried out clinical trials for AbbVie, Celgene and Janssen and has received speaking fees/attended advisory boards from AbbVie, Janssen, BMS, Almirall, LEO Pharma and Eli Lilly and reimbursement for attending a symposium from Janssen, Pfizer, Celgene and AbbVie. All funding is not personal but goes to the independent research fund of the department of dermatology of Radboud University Medical Center Nijmegen, the Netherlands. EdJ has received research grants for the independent research fund of the department of dermatology of the Radboud University Medical Center Nijmegen, the Netherlands from AbbVie, Pfizer, Novartis, Janssen Pharmaceuticals and Leo Pharma and has acted as consultant and/or paid speaker for and/or participated in research sponsored by companies that manufacture drugs used for the treatment of psoriasis including AbbVie, Janssen Pharmaceuticals, Novartis, Lily, Celgene, Leo Pharma, UCB and Almirall. All funding is not personal but goes to the independent research fund of the department of dermatology of Radboud University Medical Center Nijmegen (Radboudumc), the Netherlands. All other authors have no conflicts of interest to declare.
REFERENCES
- Coates LC, Helliwell PS. Psoriatic arthritis: state of the art review. Clin Med (Lond) 2017; 17: 65–70.
- Coates LC, Moverley AR, McParland L, Brown S, Navarro-Coy N, O’Dwyer JL, et al. Effect of tight control of inflammation in early psoriatic arthritis (TICOPA): a UK multicentre, open-label, randomised controlled trial. Lancet 2015; 386: 2489–2498.
- Mease PJ, Gladman DD, Papp KA, Khraishi MM, Thaci D, Behrens F, et al. Prevalence of rheumatologist-diagnosed psoriatic arthritis in patients with psoriasis in European/North American dermatology clinics. J Am Acad Dermatol 2013; 69: 729–735.
- Tillett W, Charlton R, Nightingale A, Snowball J, Green A, Smith C, et al. Interval between onset of psoriasis and psoriatic arthritis comparing the UK Clinical Practice Research Datalink with a hospital-based cohort. Rheumatology (Oxford) 2017; 56: 2109–2113.
- Villani AP, Rouzaud M, Sevrain M, Barnetche T, Paul C, Richard MA, et al. Prevalence of undiagnosed psoriatic arthritis among psoriasis patients: Systematic review and meta-analysis. J Am Acad Dermatol 2015; 73: 242–248.
- Alinaghi F, Calov M, Kristensen LE, Gladman DD, Coates LC, Jullien D, et al. Prevalence of psoriatic arthritis in patients with psoriasis: A systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol 2019; 80: 251–265.
- Ibrahim GH, Buch MH, Lawson C, Waxman R, Helliwell PS. Evaluation of an existing screening tool for psoriatic arthritis in people with psoriasis and the development of a new instrument: the Psoriasis Epidemiology Screening Tool (PEST) questionnaire. Clin Exp Rheumatol 2009; 27: 469–474.
- Husni ME, Meyer KH, Cohen DS, Mody E, Qureshi AA. The PASE questionnaire: pilot-testing a psoriatic arthritis screening and evaluation tool. J Am Acad Dermatol 2007; 57: 581–587.
- Gladman DD, Schentag CT, Tom BD, Chandran V, Brockbank J, Rosen C, et al. Development and initial validation of a screening questionnaire for psoriatic arthritis: the Toronto Psoriatic Arthritis Screen (ToPAS). Ann Rheum Dis 2009; 68: 497–501.
- Tinazzi I, Adami S, Zanolin EM, Caimmi C, Confente S, Girolomoni G, et al. The early psoriatic arthritis screening questionnaire: a simple and fast method for the identification of arthritis in patients with psoriasis. Rheumatology (Oxford) 2012; 51: 2058–2063.
- Audureau E, Roux F, Lons DD, Bagot M, Cantagrel A, Dernis E, et al. Psoriatic arthritis screening by the dermatologist: development and first validation of the ‘PURE-4 scale’. J Eur Acad Dermatol Venereol 2018; 32: 1950–1953.
- Urruticoechea-Arana A, Benavent D, Leon F, Almodovar R, Belinchon I, de la Cueva P, et al. Psoriatic arthritis screening: a systematic literature review and experts’ recommendations. PLoS One 2021; 16: e0248571.
- Haroon M, Kirby B, FitzGerald O. High prevalence of psoriatic arthritis in patients with severe psoriasis with suboptimal performance of screening questionnaires. Ann Rheum Dis 2013; 72: 736–740.
- Mease PJ, Gladman DD, Helliwell P, Khraishi MM, Fuiman J, Bananis E, et al. Comparative performance of psoriatic arthritis screening tools in patients with psoriasis in European/North American dermatology clinics. J Am Acad Dermatol 2014; 71: 649–655.
- van Hal TW, Van den Reek JM, Groenewoud HM, Pasch MC, Van den Hoogen FH, Wenink MH, et al. Discovery of Arthritis in Psoriasis Patients for Early Rheumatological Referral (DAPPER): protocol for a longitudinal observational study. JMIR Res Protoc 2021; 10: e31647.
- Ogdie A, Shin DB, Love TJ, Gelfand JM. Body surface area affected by psoriasis and the risk for psoriatic arthritis: a prospective population-based cohort study. Rheumatology (Oxford) 2022; 61: 1877–1884.
- Helliwell PS, Waxman R. Modification of the Psoriatic Arthritis Disease Activity Score (PASDAS). Ann Rheum Dis 2018; 77: 467–468.
- Gossec L, de WM, Kiltz U, Braun J, Kalyoncu U, Scrivo R, et al. A patient-derived and patient-reported outcome measure for assessing psoriatic arthritis: elaboration and preliminary validation of the Psoriatic Arthritis Impact of Disease (PsAID) questionnaire, a 13-country EULAR initiative. Ann Rheum Dis 2014; 73: 1012–1019.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI) – a simple practical measure for routine clinical use. Clin Exp Dermatol 1994; 19: 210–216.
- Merola JF, Tian H, Patil D, Richardson C, Scott A, Chen YH, et al. Incidence and prevalence of psoriatic arthritis in patients with psoriasis stratified by psoriasis disease severity: retrospective analysis of an electronic health records database in the United States. J Am Acad Dermatol 2022; 86: 748–757.
- van Muijen ME, van Hal TW, Groenewoud HMM, van den Reek J, de Jong E. The skin may clear but the arthritis won’t disappear: focusing on concomitant and new-onset psoriatic arthritis in a daily practice cohort of psoriasis patients on biologic therapy. Psoriasis (Auckl) 2020; 10: 29–37.
- Meer E, Merola JF, Fitzsimmons R, Love TJ, Wang S, Shin D, et al. Does biologic therapy impact the development of PsA among patients with psoriasis? Ann Rheum Dis 2022; 81: 80–86.
- Mulder MLM, van Hal TW, van den Hoogen FHJ, de Jong E, Vriezekolk JE, Wenink MH. Measuring disease activity in psoriatic arthritis: PASDAS implementation in a tightly monitored cohort reveals residual disease burden. Rheumatology (Oxford) 2021; 60: 3165–3175.
- Mease PJ, Etzel CJ, Huster WJ, Armstrong AW, Muram TM, Lisse J, et al. Evaluation of changes in skin and joint outcomes and associated treatment changes in psoriatic arthritis (psa): experience from the Corrona PsA/SpA Registry. J Rheumatol 2021; 48: 376–384.
- Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N Engl J Med 2017; 376: 957–970.
- van Winden MEC, ter Haar ELM, Groenewoud HMM, van de Kerkhof PCM, de Jong E, Lubeek SFK. Disease and treatment characteristics in geriatric psoriasis: a patient survey comparing age groups. Acta Derm Venereol 2020; 100: adv00215.
- van der Kraaij GE, Balak DMW, Busard CI, van Cranenburgh OD, Chung Y, Driessen RJB, et al. Highlights of the updated Dutch evidence- and consensus-based guideline on psoriasis 2017. Br J Dermatol 2019; 180: 31–42.
- Groll DL, To T, Bombardier C, Wright JG. The development of a comorbidity index with physical function as the outcome. J Clin Epidemiol 2005; 58: 595–602.
- Puyraimond-Zemmour D, Etcheto A, Fautrel B, Balanescu A, de Wit M, Heiberg T, et al. Associations between five important domains of health and the patient acceptable symptom state in rheumatoid arthritis and psoriatic arthritis: a cross-sectional study of 977 patients. Arthritis Care Res (Hoboken) 2017; 69: 1504–1509.
- Healy PJ, Helliwell PS. Measuring clinical enthesitis in psoriatic arthritis: assessment of existing measures and development of an instrument specific to psoriatic arthritis. Arthritis Rheum 2008; 59: 686–691.
- Fredriksson T, Pettersson U. Severe psoriasis – oral therapy with a new retinoid. Dermatologica 1978; 157: 238–244.
- Klaassen KM, van de Kerkhof PC, Bastiaens MT, Plusje LG, Baran RL, Pasch MC. Scoring nail psoriasis. J Am Acad Dermatol 2014; 70: 1061–1066.
- Rich P, Scher RK. Nail Psoriasis Severity Index: a useful tool for evaluation of nail psoriasis. J Am Acad Dermatol 2003; 49: 206–212.
- Helliwell PS, FitzGerald O, Fransen J, Gladman DD, Kreuger GG, Callis-Duffin K, et al. The development of candidate composite disease activity and responder indices for psoriatic arthritis (GRACE project). Ann Rheum Dis 2013; 72: 986–991.
- Hongbo Y, Thomas CL, Harrison MA, Salek MS, Finlay AY. Translating the science of quality of life into practice: what do dermatology life quality index scores mean? J Invest Dermatol 2005; 125: 659–664.