While dialysis is linked with prurigo nodularis, little is known about the impact of non-dialysis chronic kidney disease on prurigo nodularis. The influence of chronic kidney disease on development of prurigo nodularis was measured using the Korean National Health Insurance and National Health Screening Program data, identifying 17,295,576 individuals without prior prurigo nodularis. Chronic kidney disease severity was determined by the estimated glomerular filtration rate (in ml/min/1.73 m2) calculated from serum creatinine, and proteinuria detected with urine dipstick. Prurigo nodularis incidence during follow-up was determined. Over a median follow-up period of 9.72 years, 58,599 individuals developed prurigo nodularis, with an incidence rate of 3.59 per 10,000 person-years. Among different variables, estimated glomerular filtration rate was the strongest risk factor for prurigo nodularis. Compared with estimated glomerular filtration rate ≥ 90, estimated glomerular filtration rate 15–29 (hazard ratio 1.31, 95% confidence interval 1.05–1.62) and end-stage renal disease (hazard ratio 1.46, 95% confidence interval 1.25–1.69) were associated with higher risks. The presence of proteinuria independently increased the risk of prurigo nodularis, increased risks associated with estimated glomerular filtration rate 15–29 and end-stage renal disease, and caused risk associated with estimated glomerular filtration rate 30–59 to become significant. With differential impact of chronic kidney disease severity on the risk of prurigo nodularis, preservation of renal function would potentially translate into lower risk of prurigo nodularis.
Key words: chronic kidney disease; severity; prurigo nodularis; risk; population-based; cohort study.
Accepted Aug 16, 2022; Epub ahead of print Aug 16, 2022
Acta Derm Venereol 2022; 102: adv00781.
DOI: 10.2340/actadv.v102.2227
Corr: Hyun Jung Kim and Hyeong Sik Ahn, Department of Preventive Medicine, College of Medicine, Korea University, Inchon-ro 73, Seongbuk-gu, Seoul 02841, Korea. E-mails: moole02@naver.com; iebm.ku@gmail.com
SIGNIFICANCE
Prurigo nodularis is an under-recognized skin condition with profound impact on the patient’s quality of life. Chronic kidney disease has been linked with prurigo nodularis, yet little is known of the impact of chronic kidney disease severity, including individuals who are not undergoing dialysis, on the risk of prurigo nodularis. This nationwide cohort study is the first to suggest that chronic kidney disease severity, determined by reduced estimated glomerular filtration rates, proteinuria, and a combination of both may contribute to development of prurigo nodularis. Estimated glomerular filtration rate-associated risk of prurigo nodularis was further increased in the presence of fasting blood sugar ≥ 126 mg/dl and smoking.
INTRODUCTION
Prurigo nodularis (PN) is a chronic neurodermatitis characterized by intensely itchy nodules on the extensor surface of the limbs and trunk (1, 2). The annual incidence of PN, a distinct dermatological entity with a separate International Classification of Diseases, Tenth Revision (ICD-10) code (3), in Germany is reportedly 0.02% (4). PN is accompanied by persistent itching and is thought to develop from repetitive scratching (5) in patients with chronic pruritus from various causes (6). Epidemiological studies have determined PN to coexist with chronic liver disease, HIV, and type 2 diabetes mellitus (DM), among other diseases (1, 4, 7, 8), but the aetiological significance of the relationship is not known. An increased prevalence of PN has been observed in patients undergoing dialysis (9, 10). However, little is known of the impact of the degree of kidney dysfunction on PN, including non-dialysis CKD, in the general population.
This study investigated the influence of estimated glomerular filtration rate (eGFR) and proteinuria, both of which are determinants of CKD (11), on PN, by utilizing Korean National Health Insurance (KNHI) and National Health Screening Program (NHSP) data. Other risk factors that may affect PN, such as age, sex, fasting blood glucose, and smoking, were taken into account.
MATERIALS AND METHODS
Study design and data sources
A nationwide retrospective cohort study was conducted linking NHSP data to the NHI claims database. The Korean NHI system covers > 97% of the South Korean population (≥ 50 million) and includes all inpatient and outpatient claims data, which consists of demographic information, such as age and sex, visitation dates, diagnostic codes based on ICD-10 with the date of diagnosis, details of prescriptions, medical procedures and surgeries, and expenditure amounts (12).
The NHI provides free biennial standardized health check-ups (NHSPs) to all Koreans over 20 years of age. Each check-up includes a medical interview, postural examination, body measurements, such as height, weight, and waist circumference, chest X-ray, systolic and diastolic blood pressure (BP), regular blood and urine tests, and a questionnaire on lifestyle or medical history, including smoking and alcohol consumption. Overnight fasting is required for blood sampling, which measures creatinine, glucose, liver enzymes, and lipid profiles, and for urinalysis.
This study has been approved by the institutional review board of Incheon St Mary’s Hospital, The Catholic University of Korea (OC17ZESI0052).
Study population definition
From the database, subjects were selected who took part in the NHSP from 1 January 2009, to 31 December 2010 (baseline health examination). Exclusion criteria were: individuals with a prior PN diagnosis (ICD-10 code L28.1) and those for whom data on blood creatinine and dipstick proteinuria were missing at the baseline check-up. Based on the criteria, 17,295,576 individuals were included in the analysis. The flow chart for patient selection is shown in Fig. S1.
From the date of baseline health examination (index date), participants were followed up until development of new-onset PN (primary outcome), death, or until the end of data collection (30 August 2019), whichever came first.
Definitions of eGFR and proteinuria and information on fasting blood sugar and smoking
According to the Kidney Disease Improving Global Outcomes guidelines, CKD is classified by the level of eGFR and proteinuria (11). The eGFR measure is accepted as the best index of kidney function (11) and is calculated based on the original modification of diet in renal disease equation: eGFR (ml/min/1.73 m2)=186.3 (serum creatinine–1.154) × (age–0.203) × (0.742 for women) (13). Serum creatinine was measured by colorimetry. In accordance with the CKD staging system (11), we adopted 5 eGFR categories: eGFR ≥ 90 (normal to increased eGFR), 60–89 (mildly reduced eGFR), 30–59 (moderately reduced eGFR), 15–29 (severely reduced eGFR), and ESRD (eGFR < 15, or those who have been assigned dialysis or special-dialysis claim codes of V001 and V003 or treatment codes of O7020 for haemodialysis and O7061 for peritoneal dialysis for more than 90 days) (14). Proteinuria is a marker for kidney damage (11) and was detected using a dipstick, with the degree of proteinuria identified by a change in the colour of the reagent: “negative,” “trace (±),” “1+,” “2+,” “3+,” “4+.” For this study, we separated the dipstick proteinuria into 2 groups: “no proteinuria (−)” and “proteinuria (≥ trace).”
Individuals were categorized according to their fasting blood sugar levels by exploring cut-offs of 100 and 126 mg/dl. Individuals with fasting blood glucose level below the cut-offs were considered “normoglycaemic,” and those between 100 and 125 mg/dl as “moderate (prediabetes),” while those at or above 126 mg/dl were categorized as “hyperglycaemic (diabetes).” The smoking status of the participants, start and stop year of smoking, and the number of packs consumed per day were obtained. Based on the data, participants were categorized as “non-smoker,” “former smoker,” or “current smoker.” Period and intensity of smoking for both former and current smokers, as well as the start and stop year of smoking, were obtained, and from this information, the number of pack-years was calculated.
Study outcomes
The primary outcome of this study was incident PN, a code for which first appeared in the Korean Classification of Diseases in 1993. To identify patients with PN from the NHI database, our research team of 5 experts in the field of dermatology and epidemiology used the criteria of a PN diagnostic code (ICD-10 code L28.1) as a primary diagnosis for 3 times or more after the index date. A validation study, which reviewed 1,151 patients with an ICD-10 code L28.1, revealed a positive predictive value of 97.4% for our definition of PN (Appendix S1).
Statistical analyses
The baseline characteristics for continuous data are presented as the mean ± standard deviation (SD). Categorical data are shown as the number. Person-years were calculated for each study subject from enrolment to PN diagnosis or to the completion of the subject’s respective follow-up period. The incidence rate was estimated by dividing the number of incident-PN cases by “total person-years,” which was the sum of the person-years of all research subjects. Cases of CKD were classified based on 5 levels of eGFR and 2 groups of proteinuria. We calculated the incidence rates (number of PN events per 10,000 person-years) on whole and across the eGFR strata and dipstick proteinuria categories. The crude association between eGFR strata and PN occurrence is graphically displayed in a Kaplan–Meier curve. The incident rate (IR) ratios for PN were then calculated by dividing the IRs of PN in patients with an eGFR of 60–89, 30–59, or 15–29 by the IR of PN in patients with eGFR ≥ 90, and by dividing the IR of PN in patients with proteinuria by the IR of PN in patients without proteinuria.
With CKD as our focus, multivariable-adjusted association of eGFR, proteinuria, and their combination (eGFR × proteinuria) with incident PN were examined with Cox regression. Age, sex, body mass index, waist circumference, low-density lipoprotein, triglyceride, high-density lipoprotein, aspartate aminotransferase, alanine aminotransferase, fasting sugar level, and smoking status (Fig. S2) were included as covariates in the final model.
In addition, subgroup analyses were performed using pre-defined characteristics to identify the more vulnerable population groups. We stratified our Cox analysis by age at index date (< 60 vs ≥ 60 years), sex (male vs female), fasting blood sugar (< 126 vs ≥ 126 mg/dl) and smoking (non-smoker vs smoker) to assess their interaction with eGFR.
The statistical tests were 2-sided, and p-values < 0.05 were considered statistically significant. STATA 15.0 (StataCorp, College Station, TX, USA) software was used in the statistical analyses.
RESULTS
Characteristics of the study group
A total of 17,295,576 participants were categorized into 5 groups according to eGFR. Table I lists participant characteristics according to eGFR. The participants’ mean age was 48.3 years, and 51.5% were male. With regards to kidney function, 46.3% of the cohort were eGFR ≥ 90; 48.5% eGFR 60–89; 4.9% eGFR 30–59; 0.1% eGFR 15–29; and 0.2% ESRD (eGFR <15 or receiving dialysis).
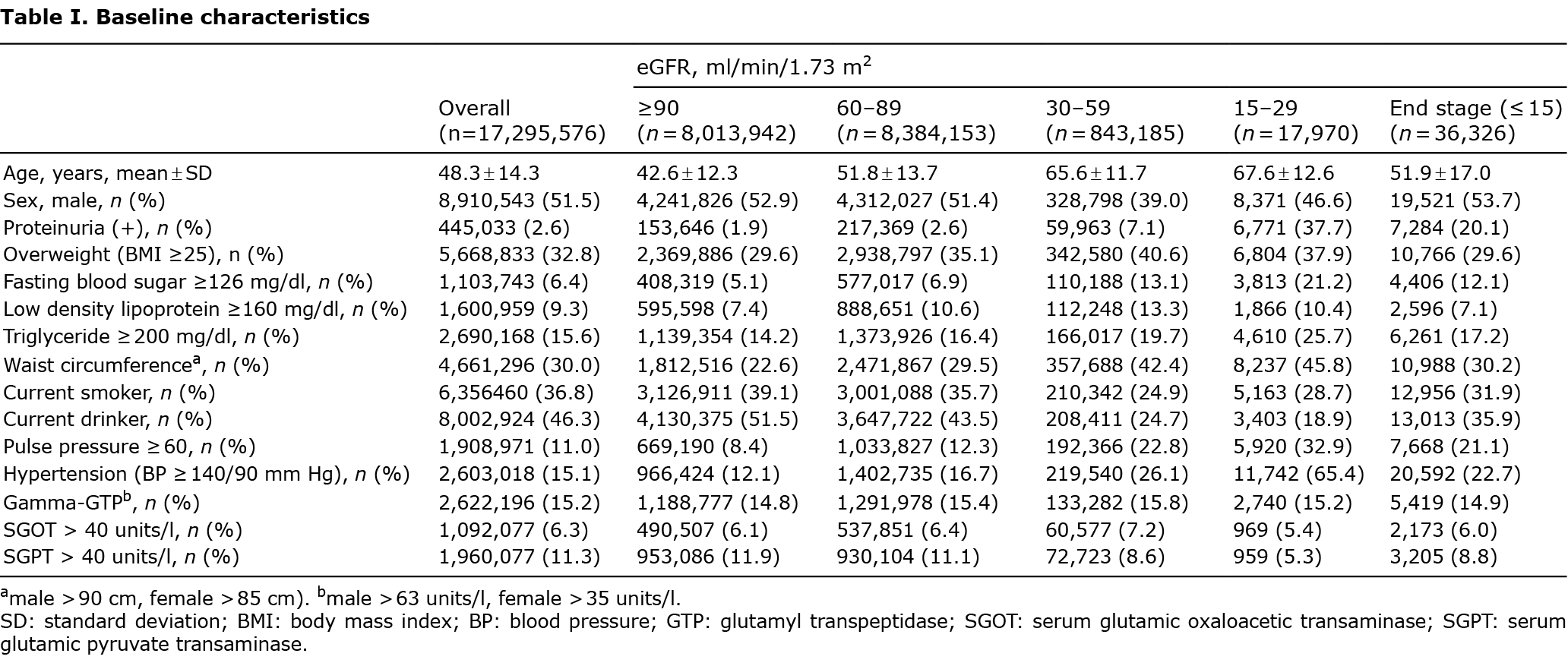
Overall prurigo nodularis incidence
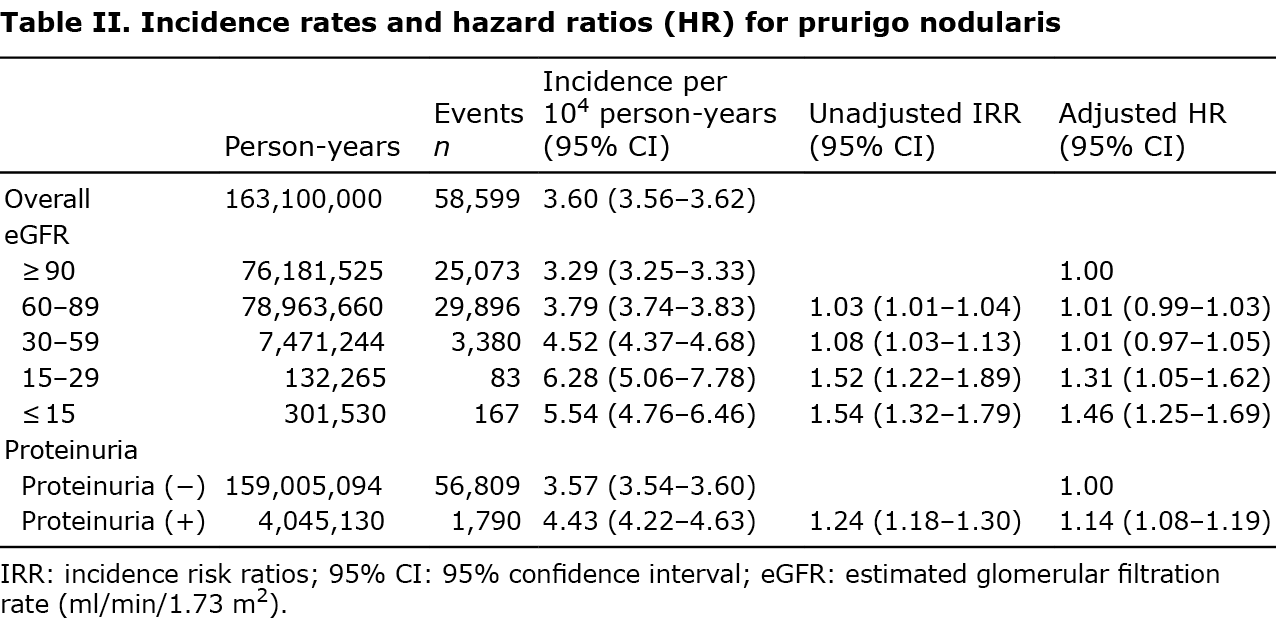
Over a median follow-up of 9.72 years (interquartile range 9.07–10.1), 58,599 individuals developed PN, with an incidence rate of 3.60 per 10,000 person-years (Table II).
Independent risk factors for prurigo nodularis
The risk of PN increased with age (≥40 years), male sex, hyperglycaemia (fasting blood sugar ≥126 mg/dL), smoking, proteinuria, and reduced eGFR, where eGFR stood out as the strongest risk factor (Fig. S2).
Association of chronic kidney disease determinants with incident prurigo nodularis
The crude PN incidence rate increased linearly as the eGFR decreased and in the presence of proteinuria (Table II, Fig. 1). The adjusted hazard ratio (HR) for PN was significantly higher in patients with eGFR 15–29, ESRD, and in the presence of proteinuria (Table II, Fig. S2).
Compared with eGFR ≥ 90, HR associated with eGFR 15–29 was 1.31 (95% confidence interval (95% CI) 1.05–1.62); and ESRD-associated HR was 1.46 (95% CI 1.25–1.69) (Table II, Fig. 1). Compared with no proteinuria, proteinuria-associated HR was 1.14 (95% CI 1.08–1.19) (Table II, Fig. S2).
The presence of proteinuria accentuated eGFR 15–29 and ESRD-associated hazards for PN, and caused the hazard associated with eGFR 30–59 to become significant (Fig. 2).
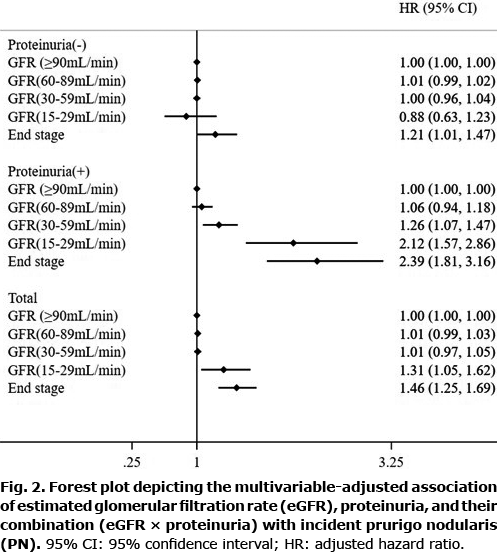
In the presence of proteinuria, the eGFR-associated HR increased greatly to 1.26 (95% CI 1.07–1.47) in patients with eGFR 30–59; 2.12 (95% CI 1.57–2.86) in those with eGFR 15–29; and 2.39 (95% CI 1.81–3.16) in those with ESRD. As for the eGFR 30–59 group, the hazard for PN was significant only in the presence of proteinuria. In the absence of proteinuria, the adjusted HR for PN was significant in those with ESRD, at 1.21 (95% CI 1.81–3.16) (Fig. 2).
Subgroup analyses
The association between eGFR and PN was assessed across 4 subgroups (Fig. 3). eGFR 15–29 and ESRD were more strongly associated with PN in men, fasting blood sugar (≥126 mg/dl), and a positive smoking history (Fig. 3).
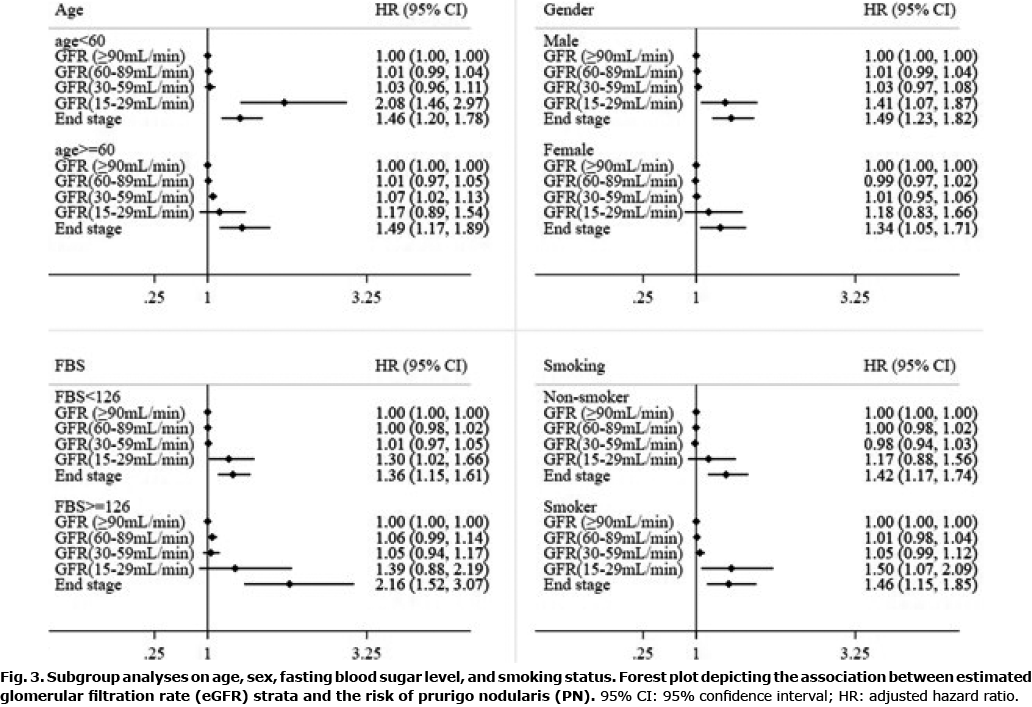
In the presence of fasting blood sugar ≥ 126 mg/dl, ESRD-associated HR was 2.16 (95% CI: 1.52–3.07) when compared with those with eGFR ≥ 90. In cases of fasting blood sugar < 126 mg/dl, the HR associated with eGFR 15–29 was 1.30 (95% CI 1.02–1.66); and ESRD-associated HR was 1.36 (95% CI 1.15–1.61) (Fig. 3).
With a smoking history, the HR associated with eGFR 15–29 was 1.50 (95% CI 1.07–2.09); and ESRD-associated HR was 1.46 (95% CI 1.15–1.83) when compared with those with eGFR ≥ 90. In cases without a history of smoking, ESRD-associated HR was 1.42 (95% CI 1.17–1.74) (Fig. 3).
DISCUSSION
The annual incidence of PN among those who took part in the NHSP in Korea was 0.036% throughout the study period, a rate that is similar to those in reports from Germany (15). The risk of developing PN was highest in the group of patients in their 60s, which is consistent with prior reports on PN being common in older adults, with a mean age of onset at 62 years (16). Males had an increased risk of incident PN compared with females, which is in line with the findings of a previous study in which 41.9% of Asian PN cases were in females (17).
This is the first study to suggest that reduced eGFR and proteinuria, both of which are incorporated in the CKD classification system (11) contribute to PN development. The risk of incident PN increased with lower eGFR, a finding suggesting a dose-response association that remained in eGFR 15–29 and ESRD categories after adjusting for covariates.
Next to being an independent risk factor for PN, proteinuria increased PN risk in patients with eGFR 15–29 and ESRD, and selectively accentuated PN risk of patients with eGFR 30–59. This is understandable, given that proteinuria is a marker of kidney damage and a strong predictor of kidney disease progression and failure (11). Multiple studies have demonstrated the benefit of renin-angiotensin-aldosterone system (RAAS) inhibition with an angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker in controlling proteinuria (18), which may help to prevent PN.
Dysregulated inflammation (19) and peripheral neuropathy (19, 20) appear to be part of the pathogenesis of PN from CKD. Of the cytokines, IL-31 is a major pruritogen and can lead to a severe itch-scratch cycle (21) that produces the characteristic pruritic nodules (22).
Uraemic neuropathy is common among dialysis and non-dialysis CKD patients (23) and is associated with an increased release of substance P (24). Substance P has also been implicated in the pathogenesis of PN (it promotes endothelial cell proliferation, contributing to nodule formation) (25) and an increased density of dermal substance P-positive sensory nerve fibres has been demonstrated in PN lesions (26). The beneficial effect of gabapentin and pregabalin in patients with PN may also be evidence of the neural origin of PN (27).
In the current study, fasting blood sugar (≥ 126 mg/dl), and a history of smoking were identified as independent risk factors for PN. Huang et al. (1) reported that patients with PN had an increased chance of having DM and hypothesized that DM-associated pruritus from skin xerosis and diabetic polyneuropathy (28) contributes to the development of PN (1). Although data on tobacco use and smoking in patients with PN are lacking, cigarette-smoke extracts have been shown to trigger expression of thymic stromal lymphopoietin (29), a cytokine that acts directly on sensory neurones to provoke itch-evoked scratching (30). In addition, smoking contributes to peripheral neuropathy, which takes part in PN pathogenesis (31).
According to subgroup analyses, the eGFR-associated risk of PN was heightened in those with a fasting blood sugar level ≥126 mg/dl, and in smokers, which indicates that CKD patients with DM or a history of smoking are the most vulnerable to developing PN. Other than their direct contribution to PN, both DM and smoking are known to induce kidney disease progression and failure via oxidative stress, hyperfiltration injury, and advanced glycosylation end products, which can further enhance PN risk (32). Smoking was an independent risk factor for PN with a dose-response nature, which suggests that its control can help PN prevention.
Finally, we found males were at a higher risk of incident PN in the presence of reduced eGFR compared with females. As underlying mechanisms, mast cell number and degranulation are known to be sex-dependent (33). In addition, uraemic neuropathy is more prevalent in male subjects (34) and women progress toward kidney failure at a slower pace compared with men (35), which explains the sex discrepancy between PN with CKD.
Limitations
This study has some limitations. First, the failure of this study to retrieve information regarding the duration of reduced eGFR and proteinuria may influence the risk of incident PN. In addition, the determination of eGFR and proteinuria was based on a single measurement, which is subject to variability, and the current study population is limited to Koreans, which is a very homogeneous population.
Despite these limitations, this study benefits from the use of nationwide population-based data with access to eGFR and proteinuria data from the NHSP database. Patients in the NHI database were followed up for 9.72 years in the current cohort study, which is the first to identify a temporal relationship between reduced eGFR/proteinuria and incident PN. Diagnoses of PN were validated and the effect of possible confounders (i.e. age, sex, fasting blood sugar ≥ 126 mg/dl, and smoking status) was controlled for and sub-analysed.
Conclusion
This is the first nationwide epidemiological study on PN in Korea to identify the differential impact of CKD severity on PN development. The data suggest that eGFR is the most significant risk factor of PN being more prominent in males, patients with fasting blood sugar ≥126 mg/dL, and smokers. The presence of proteinuria, another powerful measure for detecting and staging CKD, was also an independent risk factor of PN.
With the knowledge that CKD increases the risk of PN, preservation of renal function would potentially translate into lower risk of PN. Control of modifiable risk factors, such as hyperglycaemia, smoking, and proteinuria, may be of great benefit. Reduction in proteinuria can be achieved by using anti-hypertensives, such as angiotensin converting enzyme inhibitors.
ACKNOWLEDGEMENTS
H.S.K. would like to address special thanks to Trevi Therapeutics.
Funding. National Research Foundation of Korea (NRF) grant founded by the South Korean Government (grant number: 2020R1F1A1048238).
The data that support the findings of this study are available from the corresponding author on reasonable request.
The authors have no conflicts of interest to declare.
REFERENCES
- Huang AH, Williams KA, Kwatra SG. Prurigo nodularis: epidemiology and clinical features. J Am Acad Dermatol 2020; 83: 1559–1565.
- Zeidler C, Pereira MP, Augustin M, Spellman M, Ständer S. Investigator’s global assessment of chronic prurigo: a new instrument for use in clinical trials. Acta Derm Venereol 2021; 101: adv00401.
- Ryczek A, Reich A. Prevalence of prurigo nodularis in poland. Acta Derm Venereol 2020; 100: adv00155.
- Augustin M, Garbe C, Hagenström K, Petersen J, Pereira MP, Ständer S. Prevalence, incidence and presence of comorbidities in patients with prurigo and pruritus in Germany: A population-based claims data analysis. J Eur Acad Dermatol Venereol 2021; 35: 2270–2276.
- Kwatra SG. Breaking the Itch-scratch cycle in prurigo nodularis. N Engl J Med 2020; 382: 757–758.
- Iking A, Grundmann S, Chatzigeorgakidis E, Phan NQ, Klein D, Ständer S. Prurigo as a symptom of atopic and non-atopic diseases: aetiological survey in a consecutive cohort of 108 patients. J Eur Acad Dermatol Venereol 2013; 27: 550–557.
- Wikström K, Verkko H, Sinikumpu SP, Jokelainen J, Tasanen K, Huilaja L. Comorbidities of prurigo nodularis in Finland between 1996 and 2019. Acta Derm Venereol 2021; 101: adv00508.
- Huang AH, Canner JK, Khanna R, Kang S, Kwatra SG. Real-world prevalence of prurigo nodularis and burden of associated diseases. J Invest Dermatol 2020; 140: 480–483.e4.
- Böhme T, Heitkemper T, Mettang T, Phan NQ, Ständer S. Klinische Charakteristika und Prurigo nodularis bei nephrogenem Pruritus. Hautarzt 2014; 65: 714–720.
- Hayani K, Weiss M, Weisshaar E. Clinical findings and provision of care in haemodialysis patients with chronic itch: new results from the German epidemiological haemodialysis itch study. Acta Derm Venereol 2016; 96: 361–366.
- Levey AS, Grams ME, Inker LA. Uses of GFR and albuminuria level in acute and chronic kidney disease. N Engl J Med 2022; 386: 2120–2128.
- Lee J, Lee JS, Park SH, Shin SA, Kim K. Cohort profile: The National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int J Epidemiol 2017; 46: e15.
- Lamb EJ, Tomson CR, Roderick PJ. Estimating kidney function in adults using formulae. Ann Clin Biochem 2005; 42: 321–345.
- Choi HS, Han KD, Oh TR, Suh SH, Kim M, Kim CS, et al. Trends in the incidence and prevalence of end-stage renal disease with hemodialysis in entire Korean population: A nationwide population-based study. Medicine (Baltimore) 2021; 100: e25293.
- Ständer S, Ketz M, Kossack N, Akumo D, Pignot M, Gabriel S, et al. Epidemiology of prurigo nodularis compared with psoriasis in Germany: a Claims Database analysis. Acta Derm Venereol 2020; 100: adv00309.
- Zeidler C, Tsianakas A, Pereira M, Ständer H, Yosipovitch G, Ständer S. Chronic prurigo of nodular type: a review. Acta Derm Venereol 2018; 98: 173–179.
- Boozalis E, Tang O, Patel S, Semenov YR, Pereira MP, Stander S, et al. Ethnic differences and comorbidities of 909 prurigo nodularis patients. J Am Acad Dermatol 2018; 79: 714–719.e3.
- Ruggenenti P, Perna A, Gherardi G, Garini G, Zoccali C, Salvadori M, et al. Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet 1999; 354: 359–364.
- Williams KA, Huang AH, Belzberg M, Kwatra SG. Prurigo nodularis: pathogenesis and management. J Am Acad Dermatol 2020; 83: 1567–1575.
- Misery L, Bodere C, Genestet S, Zagnoli F, Marcorelles P. Small-fibre neuropathies and skin: news and perspectives for dermatologists. Eur J Dermatol 2014; 24: 147–153.
- Takaoka A, Arai I, Sugimoto M, Honma Y, Futaki N, Nakamura A, et al. Involvement of IL-31 on scratching behavior in NC/Nga mice with atopic-like dermatitis. Exp Dermatol 2006; 15: 161–167.
- Sekine R, Satoh T, Takaoka A, Saeki K, Yokozeki H. Anti pruritic effects of topical crotamiton, capsaicin, and a corticosteroid on pruritogen-induced scratching behavior. Exp Dermatol 2012; 21: 201–204.
- Jasti DB, Mallipeddi S, Apparao A, Vengamma B, Sivakumar V, Kolli S. A clinical and electrophysiological study of peripheral neuropathies in predialysis chronic kidney disease patients and relation of severity of peripheral neuropathy with degree of renal failure. J Neurosci Rural Pract 2017; 8: 516–524.
- El-Moneim Shoeib M, El-Hamied Yassien H, El-Mohsen Montaser B, El-Hetamy R. Substance P: does it have a role in renal pruritus? Menoufia Med J 2021; 34: 472–476.
- Choi JE, Di Nardo A. Skin neurogenic inflammation. Semin Immunopathol 2018; 40: 249–259.
- Haas S, Capellino S, Phan NQ, Böhm M, Luger TA, Straub RH, et al. Low density of sympathetic nerve fibers relative to substance P-positive nerve fibers in lesional skin of chronic pruritus and prurigo nodularis. J Dermatol Sci 2010; 58: 193–197.
- Huang AH, Canner JK, Kang S, Kwatra SG. Analysis of real-world treatment patterns in patients with prurigo nodularis. J Am Acad Dermatol 2020; 82: 34–36.
- Stefaniak AA, Krajewski PK, Bednarska-Chabowska D, Bolanowski M, Mazur G, Szepietowski JC. Itch in adult population with type 2 diabetes mellitus: clinical profile, pathogenesis and disease-related burden in a cross-sectional study. Biology (Basel) 2021; 10: 1332.
- Nakamura Y, Miyata M, Ohba T, Ando T, Hatsushika K, Suenaga F, et al. Cigarette smoke extract induces thymic stromal lymphopoietin expression, leading to T(H)2-type immune responses and airway inflammation. J Allergy Clin Immunol 2008; 122: 1208–1214.
- Wilson SR, Thé L, Batia LM, Beattie K, Katibah GE, McClain SP, et al. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell 2013; 155: 285–295.
- Clair C, Cohen MJ, Eichler F, Selby KJ, Rigotti NA. The effect of cigarette smoking on diabetic peripheral neuropathy: a systematic review and meta-analysis. J Gen Intern Med 2015; 30: 1193–1203.
- Kazancioğlu R. Risk factors for chronic kidney disease: an update. Kidney Int Suppl (2011) 2013; 3: 368–371.
- Martin CE, Clotet-Freixas S, Farragher JF, Hundemer GL. Have we just scratched the surface? A narrative review of uremic pruritus in 2020. Can J Kidney Health Dis 2020; 7: 2054358120954024.
- Jedras M, Zakrzewska-Pniewska B, Gellert R, Debowska M, Wojtaszek E, Wardyn K. [Uremic neuropathy is more frequent in male patients]. Pol Arch Med Wewn 2001; 105: 391–398 (in Polish).
- Cobo G, Hecking M, Port FK, Exner I, Lindholm B, Stenvinkel P, et al. Sex and gender differences in chronic kidney disease: progression to end-stage renal disease and haemodialysis. Clin Sci (Lond) 2016; 130: 1147–1163.