ORIGINAL REPORT
Increased Rates of Gold and Acrylate Allergy in Individuals with Fibromyalgia Tested with an Extended Dental Patch Test Series
Katharine HOPKINS1,2, Annarita ANTELMI1, Jakob DAHLIN1, Karin OLSSON1, Cecilia SVEDMAN1, Jacqueline ÅSTRAND1 and Magnus BRUZE1
Departments of 1Departments of Occupational and Environmental Dermatology, Lund University, Skåne University Hospital, Malmö, Sweden, and 2Department of Dermatology, Lund University, Skåne University Hospital, Malmö, Sweden
Fibromyalgia is a common chronic pain condition. Rates of contact allergy in individuals with fibromyalgia have not been widely studied. Systemic contact allergy can present with muscle and joint pain and general malaise. The aim of this study is to investigate contact allergy rates in individuals with fibromyalgia to the sensitizers in an extended dental series and compare with control groups. Contact allergy to gold was significantly more common in the fibromyalgia group than the dermatitis control group. When corrected for patch test system, contact allergy to gold was significantly more common in the fibromyalgia group than the dental control group. Contact allergy to hydroxyethyl methacrylate and grouped acrylates and methacrylates was significantly more common in the fibromyalgia group than the dental control group. In conclusion, individuals with fibromyalgia may have a propensity to sensitization to gold, either via an increased exposure or an alteration in the oral environment. Gold is also implicated in systemic contact dermatitis and may be a factor in elicitation of symptoms in individuals with fibromyalgia. Acrylate allergy is also common in the fibromyalgia population and may be a consequence of occupational exposure or dental treatment.
Key words: acrylates; contact allergy; delayed hypersensitivity; fibromyalgia; gold; systemic contact allergy.
SIGNIFICANCE
Fibromyalgia is a chronic common pain condition with an unknown cause. This study assessed contact allergy in individuals with fibromyalgia, compared with groups of controls. Contact allergy to gold was more common in the fibromyalgia group, and acrylate allergy was more common in the fibromyalgia group than a dental control group. It is known that muscle and joint pains can occur in gold contact allergy, and whether gold allergy could therefore have a role to play in fibromyalgia is a subject for further research. These findings are of potential significance given the individual and societal impact of fibromyalgia.
Citation: Acta Derm Venereol 2023; 103: adv22336. DOI https://doi.org/10.2340/actadv.v103.22336.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Oct 30, 2023; Published: Dec 11, 2023
Corr: Katharine Hopkins, Department of Occupational and Environmental Dermatology, Skåne University Hospital, Lund University, Jan Waldenströms Gata 16, SE-205 02 Malmö, Sweden. E-mail: katerhopkins@gmail.com
Competing interests and funding: MB is a member of the expert panel for fragrance safety (http://fragrancesafetypanel.org/). CS has received departmental funding from the International Fragrance Research Association (IFRA) for the fragrance study EFISS. KH, AA, JD, KO and JÅ have no conflicts of interest to declare.
Research Foundation of the Swedish Fibromyalgia Association, Edvard Welanders Siftelse och Finsenstiftelsen (Hudfonden).
INTRODUCTION
Fibromyalgia is a common chronic pain condition. Community studies have demonstrated a prevalence of 2%, with a female to male ratio of 2:1 (1, 2). Additional symptoms including fatigue and cognitive disturbances are frequently observed and are included in the American College of Rheumatology (ACR) diagnostic criteria (3). Fibromyalgia is partly regarded as a “centralized pain state” with origins or heightened perception of pain in the central nervous system, and an exaggerated pain response relative to the degree of nociceptive input (4). Elevated levels of proinflammatory cytokines have also been demonstrated (5, 6). Contact allergy to metals, particularly nickel allergy, has been shown to be associated with fibromyalgia (7, 8).
It is now accepted that contact allergy can present with extracutaneous symptoms as well as dermatological manifestations (9). “Systemic contact dermatitis” (SCD) refers to the elicitation of symptoms in previously sensitized individuals after systemic exposure to an allergen (10). The sensitization can occur topically, while subsequent systemic exposure can take place transcutaneously, via the mucous membranes or through systemic administration. Medications are the most common culprits, and medical implants are also implicated (11–14). Dermatitis and erythema are most commonly observed, but muscle and joint pain and general malaise can also occur (10, 12, 15). It is challenging to prove the association between extracutaneous symptoms and systemic exposure to a contact allergen. With regard to certain allergens this association has been established in case reports (15–17), but the role of exposure to metals in SCD has been demonstrated in clinical studies including provocation with gold (8, 13, 18–20).
These findings have led the authors to hypothesize that systemic contact allergy could be a contributory factor in conditions characterized by chronic pain, such as fibromyalgia, and that such symptoms could be elicited by long-term low level systemic exposure to allergens, such as in dental restorations and other allergens that people are exposed to systemically through the oral cavity.
A project with the aim of exploring contact allergy in fibromyalgia has been commenced and comprises several phases, starting with surveys assessing the prevalence of contact allergy in this group. Analysis of contact allergy to substances in the Swedish baseline series has already been reported (21).
MATERIALS AND METHODS
This study surveyed contact allergy rates in individuals with fibromyalgia to sensitizers in an extended dental series. Allergy rates in the fibromyalgia group were compared with 2 control groups: patients with dermatitis; and patients with oral symptoms suspected to be related to contact allergy.
Study group: fibromyalgia
Baseline characteristics of the study group and controls and study protocol are outlined in Fig. 1.
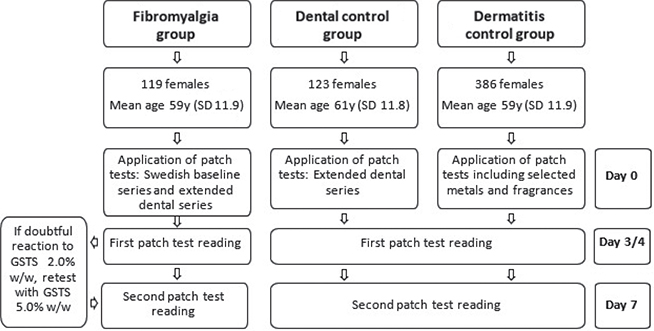
Fig. 1. Study design. y: years; SD: standard deviation; GSTS: gold sodium thiosulphate.
Volunteers were recruited from the Swedish Fibromyalgia Association (Fibromyalgiförbundet) during 2017 to 2018. Detailed descriptions of the recruitment process and study protocol have been published previously (21). A total of 120 volunteers with physician-diagnosed fibromyalgia were recruited. Only 1 of the participants was male and this subject was excluded from subsequent processing of results, for ease of analysis. The resulting 119 females had a mean ± standard deviation (SD) age of 59 ± 11.9 years.
Participants underwent patch testing with the Swedish baseline series and an extended series of 47 substances representing dental materials, including metals, acrylates, fragrance and flavouring substances, and preservatives. Two readings of patch-test reactions were performed, on day (D) 3 or 4, and D7. Prior to testing the participants answered a questionnaire on aspects of their general health and fibromyalgia, and a blood test for baseline gold levels was performed for later analysis.
Regarding gold, positive reactions can occur late and can therefore be missed in the standard time-frame for test interpretation (22). To attempt to minimize this eventuality, individuals with a “doubtful reaction” to gold sodium thiosulphate (GSTS) 2.0% on the first reading received a retest with GSTS 5.0% (23, 24). The retest was removed at 48 h and 1 reading was performed in conjunction with the D7 reading. Patients were encouraged to report any suspected late reactions that occurred after this.
Dental controls
A total of 123 females tested from 2019 to 2022 with an extended dental series due to oral symptoms suspected to be contact allergy were used as controls. Due to the relative paucity of patients tested with this series, this group was sex-matched, but not age-matched, with the fibromyalgia group (mean ± SD age 61 ± 11.8 years). Test readings in this group were performed at D3/4 and D7.
Dermatitis controls
A total of 386 females with a suspected allergic contact dermatitis who underwent patch testing during 2018 and 2019 were selected as the second control group. These patients had received patch testing with the current Swedish baseline series and selected other substances including oral allergens (selected metals and fragrances). The controls were both sex-matched and matched for age with the fibromyalgia group (mean ± SD age 59 ± 11.9 years). Readings were performed on D3/4 and D7.
Patch tests
Preparation of the test substances in the fibromyalgia group and the control groups has been described previously (21).
The test substances in the fibromyalgia group were applied using IQ chambers (Chemotechnique Diagnostics, Vellinge, Sweden). The GSTS 5.0% w/w petrolatum preparation was made at the Department of Occupational and Environmental Dermatology in Malmö, Sweden. For the 2 control groups, the testing chambers used were Finn chamber aqua (FCA) (SmartPractice Phoenix, AZ, USA).
Statistical analysis
Contact allergy rates for the individual sensitizers were compared between the 119 female participants with fibromyalgia and both control groups. Comparisons have been based on positive reactions on 1 or both of 2 readings on D3/4 and D7.
A comparison was also performed between the fibromyalgia and the dental groups regarding the number of individuals with 1 or more allergies to grouped sensitizers and the total number of positive reactions to these.
Fisher’s exact test, 2-sided was used to compare rates of allergies between the tested groups. Student’s t-test was used to calculate difference in mean age between those with and without gold allergy. Results with a p-value <0.05 were considered significant.
RESULTS
Fibromyalgia group
Positive test reactions are shown in Table I. There were 187 contact allergic reactions, out of a total 5,593 performed tests. Nickel allergy was most common (33 (27.5%) positive reactions). GSTS 2.0% was the second most common allergy (30 (25.2%)). When allergy to GSTS 5.0% and late reactions are included, this rises to 40 (33.6 %) (9 positive reactions to GSTS 5.0% at D3/4, 1 late reaction to GSTS 5.0% (on day 8)).
| Test preparation | Positive reactors (D3-4/D7a | p-value | |
| Individuals with fibromyalgia, n = 119, n (%) | Dental/oral patients, n = 123, n (%) | ||
| Potassium dichromate | 2 (1.7) | 4b (3.3) | 0.68 |
| Methyl methacrylate | 1 (0.8) | 0 (0) | 0.49 |
| Triethylene glycol dimethacrylate | 1 (0.8) | 0 (0) | 0.49 |
| Urethane dimethacrylate | 0 (0) | 0 (0) | 1 |
| Cobalt chloride hexahydrate | 7 (5.8) | 5c (4.1) | 0.57 |
| Ethylene glycol dimethacrylate | 4 (3.3) | 0 (0) | 0.057 |
| Nickel sulphate hexahydrate | 33 (27.5) | 28b (23.1) | 0.46 |
| BIS-GMA | 0 (0) | 0 (0) | 1 |
| Colophonium | 1 (0.8) | 2c (1.6) | 1 |
| N,N-dimethyl-4-toluidine | 0 (0) | 0 (0) | 1 |
| 2-Hydroxy-4-methoxy-4-methylbenzophenone | 0 (0) | 0 (0) | 1 |
| 1,4-Butanediol dimethacrylate | 1 (0) | 0 (0) | 0.49 |
| BIS-MA | 0 (0) | 0 (0) | 1 |
| Epoxy resin | 1 (0.8) | 1c (0.8) | 1 |
| Myroxylon pereirae | 15 (12.5) | 8c (6.6) | 0.13 |
| Elemental mercury 0.5% | 6 (5.0) | 7c (5.7) | 1 |
| Sodium tetrachloro palladate | 24 (20.2) | 27 (22) | 1 |
| Formaldehyde | 1 (0.8) | 3c (2.5) | 0.62 |
| 2-Hydroxyethyl methacrylate | 6 (5.0) | 0 (0) | 0.013* |
| N-ethyl-p-toluenesulphonamide | 0 (0) | 0 (0) | 1 |
| 4-Tolyldiethanolamine | 0 (0) | 0 (0) | 1 |
| Copper sulphate | 1 (0.8) | 1 (0.8) | 1 |
| Methylhydroquinone | 4 (3.3) | 0 (0) | 0.057 |
| Camphorquinone | 0 (0) | 0 (0) | 1 |
| Dimethylaminoethyl methacrylate | 1 (0.8) | 0 (0) | 0.49 |
| 1,6-Hexanediol diacrylate | 1 (0.8) | 2 (1.6) | 1 |
| Drometrizole | 1 (0.8) | 0 (0) | 0.49 |
| Tetrahydrofurfuryl methacrylate | 2 (1.7) | 0 (0) | 0.24 |
| Elemental tin | 1 (0.8) | 0 (0) | 0.49 |
| Elemental titanium | 0 (0) | 0 (0) | 1 |
| Calcium titanate | 0 (0) | 0 (0) | 1 |
| Silver sulphate | 0 (0) | 0c (0) | 1 |
| Ammonium hexachloroplatinate | 0 (0) | 0 (0) | 1 |
| Glutaraldehyde | 1 (0.8) | 1 (0.8) | 1 |
| Titanium nitride | 0 (0) | 0 (0) | 1 |
| Elemental mercury 1.6% | 9 (7.6) | 13 (10.6) | 0.50 |
| Sodium metabisulphite | 6 (5.0) | 2 (1.6) | 0.16 |
| BIS-EMA | 0 (0) | 1 (0.8) | 1 |
| Thiomersal | 6 (5.0) | 4c (3.3) | 0.53 |
| Aluminium chloride hexahydrate | 3 (2.5) | 0 (0) | 0.12 |
| Gold sodium thiosulphate 2.0% | 30 (25.2) | 20b (16.5) | 0.11 |
| Canada balsam | 0 (0) | 3b (2.5) | 0.25 |
| Eugenol | 2 (1.7) | 0b (0) | 0.24 |
| Carvone | 3 (2.5) | 9 (7.3) | 0.14 |
| Cinnamal | 1 (0.8) | 2 (1.6) | 1 |
| Oxidized linalool | 8 (6.7) | 15 (12.2) | 0.19 |
| Oxidized limonene | 5 (4.2) | 16 (13.0) | 0.021† |
| Total | 187 | 174 | 0.36 |
| aResult on either first (days (D) 3–4) or second (day (D) 7) reading, b121 patients tested, c122 patients tested. | |||
| BIS-GMA: bisphenol A glycerolate dimethyacrylate; BIS-EMA: 2,2-bis (4-(2-methacryloxyethoxy)phenyl)propane; BIS-MA: bisphenol A dimethacrylate. Statistically significant p-values in bold. *Significantly more reactions in fibromyalgia group, †Significantly more reactions in dental control group. | |||
The mean ± SD age of the gold-allergic individuals was 55 ± 12.7 years and of those without gold allergy was 60 ± 11.2 years (p = 0.034).
Fibromyalgia group vs dental control group
The results are shown in Table I. There was no difference in the number of reactions in the 2 groups. (p = 0.36). There were significantly more reactions to 2-hydroxyethyl methacrylate (p = 0.013) in the fibromyalgia group. Reactions to limonene (p = 0.021) were overrepresented in the dental group.
Comparisons of numbers of individuals with positive reactions and the total number of positive reactions to grouped substances are shown in Table II. There were significantly more individuals with at least 1 allergy to acrylates, methacrylates and substances used in the acrylate polymerization process in the fibromyalgia group (p = 0.016) as well as significantly more individual reactions to acrylates and methacrylates (p = 0.0001, p = 0.0012 when polymerization substances included).
| Group of sensitizers | Individuals with fibro-myalgia (n = 119) n (%) | Dental/oral patients (n = 123) n (%) | p-value |
| Metalsa | |||
| ≥1 CA | 60 (50.4) | 56 (45.5) | 0.52 |
| Total number contact allergy | 118 | 105 | 0.58 |
| Fragrancesb | |||
| ≥1 contact allergy | 24 (20.2) | 34 (27.6) | 0.18 |
| Total number contact allergy | 35 | 55 | 0.052 |
| Acrylates and methacrylatesc | |||
| Total number contact allergy | 17 | 3 | 0.0012 |
| Acrylates, methacrylates and associated substancesd | |||
| ≥1 contact allergy | 12 (10.1) | 3 (2.4) | 0.016 |
| Total number contact allergy | 23 | 4 | 0.0001 |
| Preservativese | |||
| ≥1 contact allergy | 13 (10.9) | 9 (7.3) | 0.38 |
| Total number contact allergy | 14 | 10 | 0.41 |
| aChromium, cobalt, nickel, mercury 0.5%, palladium, copper, tin, elemental titanium, calcium titanate, silver, platinum, titanium nitride, mercury 1.6%, aluminium, gold. bColophony, Myroxolon pereirae, Canada balsam, eugenol, carvone, cinnamal, oxidized linalool, oxidized limonene. cMethyl methacrylate, triethylene glycol dimethacrylate, urethane dimethylacrylate, ethylene glycol dimethacrylate, bisphenol A glycerolate dimethyacrylate, butanediol methacrylate, bisphenol A dimethacrylate, hydroxyethyl methacrylate, dimethylaminoethyl acrylate, hexanediol diacrylate, tetrahydrofurfuryl methacrylate, 2,2-bis (4-(2-methacryloxyethoxy)phenyl)propane. dAcrylates and methacrylates as above, N,N-dimethyl-4-toludine, 2-hydroxy-4-methoxy-4-methylbenzophenone, epoxy resin, N-ethyl-p-toluenesulphonamide, 4-tolyldiethanol amine, methylhydroquinone, camphorquinone, drometrizole. eFormaldehyde, thiomersal, glutaraldehyde, sodium metabisulphite.Statistically significant p-values in bold. | |||
Fibromyalgia group vs dermatitis group
The results are shown in Table III. There was no significant difference in the total rates of allergy observed between the groups (p = 0.28). Allergy to GSTS 2.0% was significantly higher in the fibromyalgia group (p = 0.043).
| Test preparation | Positive reactors (n) (D3–4/D7)a | p-value | |
| Individuals with fibromyalgia, (n = 119) n (%) | Dermatitis patients, (n = 386) n (%) | ||
| Sodium tetrachloro palladite | 24 (20.2) | 70 (18.2) | 0.59 |
| Aluminium chloride hexahydrate | 3 (2.5) | 2 (0.5) | 0.088 |
| Gold sodium thiosulphate 2.0% | 30 (25.2) | 64 (16.8) | 0.043 |
| Eugenol | 2 (1.7) | 1 (0.3) | 0.14 |
| l-carvone | 3 (2.5) | 7 (1.8) | 0.71 |
| Cinnamal | 1 (0.8) | 6 (1.6) | 1 |
| Oxidized linalool | 8 (6.7) | 32 (8.3) | 0.70 |
| Oxidized limonene | 5 (4.2) | 33 (8.6) | 0.16 |
| Total | 76 (8.0) | 215 (7.0) | 0.28 |
| aResult on either first (days 3–4) or second (day 7) reading. | |||
DISCUSSION
This study, in conjunction with our previous paper assessing contact allergy rates to the Swedish baseline series (21), is to our knowledge, the first where contact allergy frequencies in fibromyalgia are established and compared with sex- and age-matched patients.
Interestingly, there was no difference in the rates of total allergies between the fibromyalgia group and the patients investigated for oral and perioral symptoms (p = 0.36). One may expect the difference between the groups to be greater; a group undergoing investigation specifically for contact allergy would be expected to have higher rates of allergy than a group drawn from the general population with a systemic condition rather than a dermatological disease. Our previous findings on contact allergy to the Swedish baseline series in individuals with fibromyalgia demonstrated allergy rates that were more akin to those in a control group of patients with dermatitis than the general population (21). This previous study also indicated that the fibromyalgia group is not overrepresented in all groups of common sensitizers, favouring the hypothesis that these individuals have a different exposure profile to the general population.
Upon analysis of individual sensitizers, allergy to gold in the fibromyalgia group was demonstrably higher than has previously been reported in general population testing. Positive patch-test reactions to GSTS are common, with frequencies of approximately 10% reported when using GSTS 0.5% as the test preparation (25–28). An analysis of testing performed with GSTS 2.0% demonstrated a prevalence of gold allergy of 14% amongst individuals with suspected allergic contact dermatitis (29). In this study, the fibromyalgia group had a positive reaction rate of 33.6% to gold when including GSTS 5.0% and late reactions to GSTS 5.0%. Such high levels of gold allergy have previously been demonstrated in individuals who have had systemic exposure to gold coronary stents (37%) (19).
The mean age of the female fibromyalgia patients with gold allergy in this study was 55 years, somewhat older than the mean of 44.8 years (males and females) and 37 years (females only) demonstrated in previous studies assessing the prevalence in patients with contact dermatitis (28, 30). This can be explained by the older age group assessed in our study. Prevalence of gold allergy has decreased in the dermatitis population in southern Sweden in recent years (29), which can be explained by the declining use of gold alloys in dental restorations due to increased costs and development of materials with better mechanical properties (31).
Prevalence of gold allergy was significantly higher in the fibromyalgia group than in the dermatitis control group (p = 0.038). Initial analysis demonstrated a numerical overrepresentation of gold allergy in the fibromyalgia group compared with the dental control group (25.2% vs16.5%), although this was not significant (p = 0.11). However, there was a difference in testing systems between the different groups; IQ chambers in the fibromyalgia group and FCA in the control groups. Regarding GSTS 2.0 %, this results in the delivery of 25 mg and 20 mg of test material, respectively. An analysis assessing whether the different test methods vary in the detection rate of gold allergy demonstrated that the FCA trace 20.3% more allergy to GSTS 2.0% than IQ chambers (32), suggesting that the rate of allergy to GSTS 2.0% in the fibromyalgia group could have been underestimated. Using FCA could have detected up to 6 additional individuals with gold allergy, giving a total prevalence of 30.3%. When applied to comparison with the control groups this gives a significantly higher prevalence of allergy to GSTS 2.0% in the fibromyalgia group compared with both the dental group (p = 0.015) and the dermatitis control group (p = 0.001).
The findings related to gold allergy are of interest given the particularly enigmatic nature of gold as an allergen. There have previously been doubts raised as to the relevance of positive test reactions to gold due to the propensity of metal test preparations to cause irritant reactions, and the poor correlation between positive test reactions and cutaneous exposure to gold in the form of gold jewellery (27). However, gold allergy is overrepresented in those with dental gold or gold coronary stents (13, 18, 19, 22, 27), suggesting that the intraoral and vascular environments are more favourable than the cutaneous environment in eliciting ionisation and thus haptenization of gold (33).
In our previous study it was demonstrated that individuals with fibromyalgia had increased rates of contact allergy to fragrance sensitizers present in flavouring and dental substances compared with the general population, with allergies to fragrance mix I and Myroxolon pereirae being overrepresented. There was no overrepresentation of other groups of allergies in the fibromyalgia group (21). Myroxolon pereirae allergy was also overrepresented in the fibromyalgia group in the present study, although this was not statistically significant. Allergy to other fragrances including oxidized limonene, oxidized linalool and carvone was overrepresented in the dental control group compared with the fibromyalgia group with frequency of oxidized limonene allergy being significant (p = 0.021). These findings are not surprising given that the dental control group comprised of patients being investigated for oral symptoms, and fragrance allergy is a relatively common finding in such instances, with the above substances being used frequently in toothpaste and cosmetics (34–36).
Regarding acrylates (including methacrylates) and substances involved in acrylate processing, significantly more allergies were demonstrated in the fibromyalgia group, both to an individual allergen (hydroxyethyl methacrylate) and the grouped allergens. Rates of allergy to ethylene glycol dimethacrylate and methylhydroquinone, a stabilizer used in acrylic monomers, were also elevated in the fibromyalgia group, although this was not significant. Rates of acrylate allergy in the fibromyalgia group were considerably higher than previously reported, with 10.1% of participants demonstrating at least 1 acrylate allergy. Previous population testing has demonstrated a frequency of 2.3%, with allergies in dental personnel being somewhat higher at 5.8% (37, 38). The individuals with fibromyalgia would not be expected to have a level of exposure to acrylates greater than those with an occupational exposure, hence one has to query if individuals with fibromyalgia present a risk group for sensitization to such substances, or whether exposure to acrylates is increased in this group, potentially via occupational or dental exposure.
The combination of the above findings in the fibromyalgia group suggests that these individuals may have a propensity to developing allergy to gold and other substances where sensitization can occur via the oral mucosa. Whether this exposure profile is secondary to a factor caused by the condition itself (for example, if the oral environment in these individuals is particularly favourable for sensitization), or whether it is lifestyle related in this group cannot be established in retrospect and remains to be explored.
An association between contact allergies to metals and inflammatory and rheumatological diseases has been reported previously. Patients with systemic lupus erythematosus, rheumatoid arthritis and Sjögren’s syndrome have been shown to have an increased frequency of delayed hypersensitivity to metals including mercury, gold and nickel (7, 39, 40). Regarding other chronic pain syndromes, an increased prevalence of nickel allergy has been observed in chronic fatigue syndrome (CFS) (41, 42). Although patients with fibromyalgia often report subjective cutaneous symptoms and sensitivities, there are very few studies assessing contact allergy in this group. A study performed using an in vitro lymphocyte transformation test demonstrated a high level of allergy to dental metals in patients with fibromyalgia compared with controls (43), but research focusing specifically on patch testing of metals in patients with fibromyalgia is lacking.
The clinical presentation of gold allergy is heterogeneous, particularly in the context of SCD. Reports demonstrate that patients with confirmed gold allergy can experience cutaneous and generalised reactions upon parenteral administration of gold (44, 45). Elevated levels of inflammatory cytokines have also been observed in those with gold allergy. Further evidence for the potential role of gold in SCD includes gold-allergic patients with gold-coated coronary stents demonstrating an increased risk of in-stent restenosis (13). Furthermore, a positive correlation has been shown between gold allergy, number of gold dental restorations and oral lichenoid lesions (18, 46). An interesting case report outlined a case of perianal dermatitis and general malaise in a patient with gold allergy who was using a homeopathic oral gold preparation (15).
The question remains as to whether there is a potential clinical significance in terms of SCD to gold related to fibromyalgia (the “elicitation hypothesis”). Very limited previous studies with low patient numbers have demonstrated that patients with CFS/fibromyalgia overlap syndromes and metal allergy report an improvement in systemic symptoms upon replacement of dental restorations with non-metallic alternatives (43, 47). A multitude of oral symptoms have been described in the context of fibromyalgia, including xerostomia, glossodynia and dysgeusia (48, 49), but presence of clinical oral lesions in patients with fibromyalgia has, as yet, not been shown to have increased prevalence compared with controls (48).
The strengths of this study include the high number of participants and controls. The patch-test readings were performed by experienced dermatologists who were blinded to the participants’ medical history and questionnaire responses. The same group of dermatologists assessed the patch-test readings of the control groups. Regarding limitations, the control group of dental patients was not matched for age due to low numbers of patients tested with this series. The patch-test system also differed between the study individuals and the controls, although the correction method for gold outlined above has mitigated for this to some extent.
In conclusion, this survey of prevalence of contact allergy in fibromyalgia has demonstrated surprising results. Compared with controls, patients with fibromyalgia appear to not only have a tendency towards development of contact allergy, but there also appears to be a predilection for certain specific allergens in this patient group, namely those that can act as sensitizers through the oral and perioral route. In addition, in the case of gold, we have seen a high level of allergy to an allergen previously reported to be implicated in SCD. The natural hypothesis generated from the results is whether these associations have a clinical significance, either as a contributing factor in fibromyalgia, or as a consequence of exposure. Further exploration of this is planned, with results from questionnaires obtained in this study and clinical dental examinations. The initial findings from this study open a potential new sphere of research into this debilitating and prevalent condition.
ACKNOWLEDGEMENTS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethical approval: Approval was obtained from the regional ethics committee in Lund, Sweden (2017/487). The study was performed in accordance with the Declaration of Helsinki.
REFERENCES
- Wolfe F, Ross K, Anderson J, Russell IJ, Hebert L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum 1995; 38: 19–28.
- Vincent A, Lahr BD, Wolfe F, Clauw DJ, Whipple MO, Oh TH, et al. Prevalence of fibromyalgia: a population-based study in Olmsted County, Minnesota, utilizing the Rochester Epidemiology Project. Arthritis Care Res (Hoboken) 2013; 65: 786–792.
- Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Häuser W, Katz RL, et al 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum 2016; 46: 319–29.
- Clauw DJ. Fibromyalgia: a clinical review. JAMA 2014; 311: 1547–1555.
- Gerdle B, Ghafouri B, Ghafouri N, Bäckryd E, Gordh T. Signs of ongoing inflammation in female patients with chronic widespread pain. Medicine (Baltimore) 2017; 96: e6130.
- Mendieta D, Cruz-Aguilera DLD la, Barrera-Villalpando MI, Becerril-Villanueva E, Arreola R, Hernández-Ferreira E, et al. IL-8 and IL-6 primarily mediate the inflammatory response in fibromyalgia patients. J Neuroimmunol 2016; 290: 22–25.
- Bjørklund G, Dadar M, Aaseth J. Delayed-type hypersensitivity to metals in connective tissue diseases and fibromyalgia. Environ Res 2018; 161: 573–579.
- Marcusson JA. Contact allergies to nickel sulfate, gold sodium thiosulfate and palladium chloride in patients claiming side-effects from dental alloy components. Contact Dermatitis 1996; 34: 320–323.
- Pongpairoj K, Ale I, Andersen KE, Bruze M, Diepgen TL, Elsner PU, et al. Proposed ICDRG Classification of the Clinical Presentation of Contact Allergy. Dermatitis 2016; 27: 248–258.
- Kulberg A, Schliemann S, Elsner P. Contact dermatitis as a systemic disease. Clin Dermatol 2014; 32: 414–419.
- de Groot AC. Systemic allergic dermatitis (systemic contact dermatitis) from pharmaceutical drugs: a review. Contact Dermatitis 2022; 86: 145–164.
- Möller H, Ohlsson K, Linder C, Björkner B, Bruze M. The flare-up reactions after systemic provocation in contact allergy to nickel and gold. Contact Dermatitis 1999; 40: 200–204.
- Ekqvist S, Svedman C, Lundh T, Möller H, Björk J, Bruze M. A correlation found between gold concentration in blood and patch test reactions in patients with coronary stents. Contact Dermatitis 2008; 59: 137–142.
- Veien NK. Systemic contact dermatitis. Int J Dermatol 2011; 50: 1445–1456.
- Malinauskiene L, Isaksson M, Bruze M. Systemic contact dermatitis in a gold-allergic patient after treatment with an oral homeopathic drug. J Am Acad Dermatol 2013; 68: e58.
- Andersen KE, Hjorth N, Menné T. The baboon syndrome: systemically-induced allergic contact dermatitis. Contact Dermatitis 1984; 10: 97–100.
- Erdmann SM, Sachs B, Merk HF. Systemic contact dermatitis from cinchocaine. Contact Dermatitis 2001; 44: 260–261.
- Ahnlide I, Ahlgren C, Björkner B, Bruze M, Lundh T, Möller H, et al. Gold concentration in blood in relation to the number of gold restorations and contact allergy to gold. Acta Odontol Scand 2002; 60: 301–305.
- Ekqvist S, Svedman C, Möller H, Kehler M, Pripp CM, Björk J, et al. High frequency of contact allergy to gold in patients with endovascular coronary stents. Br J Dermatol 2007; 157: 730–738.
- Möller H, Björkner B, Bruze M. Clinical reactions to systemic provocation with gold sodium thiomalate in patients with contact allergy to gold. Br J Dermatol 1996; 135: 423–427.
- Bruze M, Hopkins K, Dahlin J, Olsson K, Åstrand J, Svedman C, et al. Increased rates of fragrance allergy in fibromyalgia individuals tested with the Swedish baseline patch test series. J Eur Acad Dermatol Venereol 2023; 37: 104–113.
- Svedman C, Tillman C, Gustavsson CG, Möller H, Frennby B, Bruze M. Contact allergy to gold in patients with gold-plated intracoronary stents. Contact Dermatitis 2005; 52: 192–196.
- Johansen JD, Aalto-Korte K, Agner T, Andersen KE, Bircher A, Bruze M, et al. European Society of Contact Dermatitis guideline for diagnostic patch testing – recommendations on best practice. Contact Dermatitis 2015; 73: 195–221.
- Bruze M, Condé-Salazar L, Goossens A, Kanerva L, White IR. Thoughts on sensitizers in a standard patch test series. The European Society of Contact Dermatitis. Contact Dermatitis 1999; 41: 241–250.
- Sabroe RA, Sharp LA, Peachey RD. Contact allergy to gold sodium thiosulfate. Contact Dermatitis 1996; 34: 345–348.
- Björkner B, Bruze M, Möller H. High frequency of contact allergy to gold sodium thiosulfate. An indication of gold allergy? Contact Dermatitis 1994; 30: 144–151.
- Bruze M, Edman B, Björkner B, Möller H. Clinical relevance of contact allergy to gold sodium thiosulfate. J Am Acad Dermatol 1994; 31: 579–583.
- Tsuruta K, Matsunaga K, Suzuki K, Suzuki R, Akita H, Washimi Y, et al. Female predominance of gold allergy. Contact Dermatitis 2001; 44: 55–56.
- Björk A-K. Patch testing with metals with focus on gold. Lund: Lund University; 2017.
- McKenna KE, Dolan O, Walsh MY, Burrows D. Contact allergy to gold sodium thiosulfate. Contact Dermatitis 1995; 32: 143–146.
- Roach M. Base metal alloys used for dental restorations and implants. Dent Clin North Am 2007; 51: 603–627, vi.
- Antelmi A, Dahlin J, Hopkins K, Svedman C, Bruze M. Contact allergy to gold simultaneously patch tested in two different chambers. Contact Dermatitis 2023; 89: 54–56
- Svedman C, Gruvberger B, Dahlin J, Persson L, Möller H, Bruze M. Evaluation of a method for detecting metal release from gold; cysteine enhances release. Acta Derm Venereol 2013; 93: 577–578.
- Sainio EL, Kanerva L. Contact allergens in toothpastes and a review of their hypersensitivity. Contact Dermatitis 1995; 33: 100–105.
- Kroona L, Warfvinge G, Isaksson M, Ahlgren C, Dahlin J, Sörensen Ö, et al. Quantification of l-carvone in toothpastes available on the Swedish market. Contact Dermatitis 2017; 77: 224–230.
- Ahlgren C, Axéll T, Möller H, Isaksson M, Liedholm R, Bruze M. Contact allergies to potential allergens in patients with oral lichen lesions. Clin Oral Investig 2014; 18: 227–237.
- Goon ATJ, Isaksson M, Zimerson E, Goh CL, Bruze M. Contact allergy to (meth)acrylates in the dental series in southern Sweden: simultaneous positive patch test reaction patterns and possible screening allergens. Contact Dermatitis 2006; 55: 219–226.
- Teik-Jin Goon A, Bruze M, Zimerson E, Goh CL, Isaksson M. Contact allergy to acrylates/methacrylates in the acrylate and nail acrylics series in southern Sweden: simultaneous positive patch test reaction patterns and possible screening allergens. Contact Dermatitis 2007; 57: 21–27.
- Stejskal V, Reynolds T, Bjørklund G. Increased frequency of delayed type hypersensitivity to metals in patients with connective tissue disease. J Trace Elem Med Biol 2015; 31: 230–236.
- Rachmawati D, Muris J, Scheper RJ, Rustemeyer T, Kleverlaan CJ, Feilzer AJ, et al. Continuing the quest for autoimmunity due to oral metal exposure. Autoimmunity 2015; 48: 494–501.
- Sterzl I, Procházková J, Hrdá P, Bártová J, Matucha P, Stejskal VD. Mercury and nickel allergy: risk factors in fatigue and autoimmunity. Neuro Endocrinol Lett 1999; 20: 221–228.
- Marcusson JA, Lindh G, Evengård B. Chronic fatigue syndrome and nickel allergy. Contact Dermatitis 1999; 40: 269–272.
- Stejskal V, Ockert K, Bjørklund G. Metal-induced inflammation triggers fibromyalgia in metal-allergic patients. Neuro Endocrinol Lett 2013; 34: 559–565.
- Möller H, Larsson A, Björkner B, Bruze M, Hagstam A. Flare-up at contact allergy sites in a gold-treated rheumatic patient. Acta Derm Venereol 1996; 76: 55–58.
- Svensson A, Möller H, Björkner B, Bruze M, Leden I, Theander J, et al. Rheumatoid arthritis, gold therapy, contact allergy and blood cytokines. BMC Dermatol 2002; 2: 2.
- Ahlgren C, Bruze M, Möller H, Gruvberger B, Axéll T, Liedholm R, et al. Contact allergy to gold in patients with oral lichen lesions. Acta Derm Venereol 2012; 92: 138–143.
- Stejskal V. Metals as a common trigger of inflammation resulting in non-specific symptoms: diagnosis and treatment. Isr Med Assoc J 2014; 16: 753–758.
- Rhodus NL, Fricton J, Carlson P, Messner R. Oral symptoms associated with fibromyalgia syndrome. J Rheumatol 2003; 30: 1841–1845.
- Balasubramaniam R, Laudenbach JM, Stoopler ET. Fibromyalgia: an update for oral health care providers. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007; 104: 589–602.