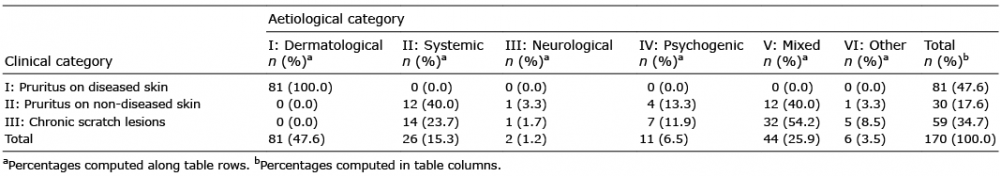
Table I. Classification of chronic pruritus in 170 patients participating in the study according to clinical and aetiological categories
1Department of Dermatology, Inselspital, Bern University Hospital, University of Bern, Switzerland, 2Centro Studi GISED, Bergamo, Italy, and 3Graduate School for Cellular and Biomedical Sciences, University of Bern, Bern, Switzerland
#These authors contributed equally to the study.
Chronic pruritus profoundly affects patients’ quality of life. The objective of this retrospective cross-sectional study was to characterize patients with chronic pruritus and identify patterns, in order to delineate a better diagnostic approach. Both semantic connectivity map and classical analysis were applied, linking demographic, clinical, laboratory and histopathological data with clinical and aetiological categories of 170 patients with chronic pruritus (median age 72 years, 58.2% women). The semantic map showed clinical categories separated in different hubs associated with distinct patterns concerning sex, aetiology, laboratory findings, and pharmacological treatment. Diabetes, diagnosis of cancer and psychiatric comorbidities were linked with certain clinical categories. Skin eosinophilia was a common finding of chronic pruritus, on both diseased and non-diseased skin. High frequencies of patients with chronic pruritus taking anti-arrhythmics, beta-blockers and AT-II receptor antagonists were noticed among those with underlying systemic, neurological and psychiatric diseases. This study provides a complex analysis of chronic pruritus and thus basic principles for a clinical work-up.
Key words: chronic pruritus; classification; semantic map; eosino-philia.
Accepted Jan 9, 2020; Epub ahead of print Jan 17, 2020
Acta Derm Venereol 2020; 100: adv00068.
Corr: Anna Rammlmair, Department of Dermatology, Inselspital, Bern University Hospital, CH-3010 Bern, Switzerland. E-mail: anna.rammlmair@insel.ch
This retrospective cross-sectional study is aimed at characterizing patients with chronic pruritus and identifying patterns and interactions of factors with pruritus. The semantic map analysis showed clinical categories separated in different hubs associated with distinct patterns concerning sex, aetiology, laboratory findings and pharmacological treatment. Diabetes, diagnosis of cancer and psychiatric comorbidities were linked with distinct clinical categories of itch. Skin eosinophilia was a common finding of chronic pruritus on both diseased and non-diseased skin. This study provides a complex analysis of the characteristics of patients with chronic pruritus, as well as basic principles for a practical clinical work-up and management.
Pruritus was defined in 1660 by Samuel Hafenreffer as an unpleasant sensation leading to scratching. Acute pruritus has been distinguished from chronic pruritus (CP), which is defined as pruritus lasting for 6 weeks or more (1, 2). CP has been found more frequently in women than men, and its incidence increases with age. Depending on the severity and chronicity, CP may profoundly affect patients’ quality of life (3). In a recent study, CP was shown to be as debilitating as chronic pain (4). Pruritus may involve either the entire skin (generalized pruritus) or only particular areas (localized pruritus).
The current Clinical Classification of Itch of the International Forum for the Study of Itch focuses on clinical signs, in particular the presence or absence of primary and secondary skin lesions. Thus, 3 clinical categories (CC) have been defined: pruritus on diseased (inflamed) skin (CC-I), pruritus on non-diseased (non-inflamed) skin (CC-II), and pruritus presenting with severe chronic secondary scratch lesions, such as prurigo nodularis (CC-III). Furthermore, underlying conditions causing itch have been categorized into 6 categories: dermatological diseases (aetiological category (EC)-I); systemic diseases including diseases associated with pregnancy and drug-induced pruritus (EC-II); neurological and psychiatric diseases (EC-III, EC-IV); more than one cause of pruritus (category “mixed”, EC-V); and underlying disease not identified (category “others”, EC-VI) (2).
Data on CP as a common symptom and its epidemiological, clinical and histopathological context remain scarce. This study therefore aimed at characterizing patients referred to a tertiary university centre for evaluation of CP by applying the above classification of itch. Based on this classification, as well as on demographic, clinical, laboratory, and histopathological data, the study sought to identify patterns potentially helpful as diagnostic tools and to improve the management of patients.
Study design
This was a retrospective cross-sectional study including all consecutive inpatients with CP as main diagnosis referred to and diagnosed at the Department of Dermatology, Inselspital, University Hospital of Bern between January 2010 and December 2015. Cases were retrieved by searching the keywords “chronic pruritus” and “itch” in the lists of diagnoses of the department’s electronic medical report archives. The electronic files of all cases were reviewed, and relevant retrospective data were extracted systematically using a standardized clinical report form (Table SI). The study was approved by the Ethics Committee of the Canton of Bern, Switzerland. General informed consent had been obtained from all patients prior to the study. All clinical investigations were conducted according to the principles of the Declaration of Helsinki.
Collected data
Collected data included demographics, patients’ history, presence of comorbidities (such as diabetes mellitus, arterial hypertension, hyperlipidaemia, ulcer, chronic liver diseases (CLD), chronic kidney diseases (CKD), cancer and psychiatric condition), previous and current treatments, clinical presentation, dermatopathological findings, laboratory results, radiological imaging and patch test results, where available. In particular, normal and abnormal ranges of laboratory parameters were defined according to the criteria listed in the footnote to Table SII.
Based on these data, patients with CP were classified according to their clinical picture and history, as well as underlying diseases according to the International Forum for the Study of Itch (2). Since the number of patients was limited, groups EC-III and IV as well as EC-V and VI were merged for statistical purposes.
Statistical analysis
Data were presented as numbers with percentages, or medians with interquartile ranges (IQR) for categorical and continuous variables, respectively. Differences in the distribution of factors across strata of clinical and aetiological classification of CP were assessed by means of Kruskal–Wallis and Pearson’s χ2 tests or Fisher’s exact test where required, for continuous and categorical variables, respectively. Before starting the study, it was estimated that, with 170 patients, the study would be able to detect a minimum effect size of 0.26 for continuous variables (as measured by Cohen’s f) and of 0.27 for categorical variables (as measured by Cohen’s w), by considering 4 groups or fewer overall (1-way analysis of variance (ANOVA) and Pearson’s χ2 tests, α = 0.05, β = 0.20). These correspond to a medium effect size according to Cohen’s guidelines (5). All tests were considered significant at p-value < 0.05. Analyses were performed with SPSS ver. 20 (IBM Corp, Armonk, NY, USA).
Semantic map analysis
Associations among variables that were selected based on their clinical relevance and statistical significance (i.e. sex, age, duration of disease, presence of atopy, cancer occurrence within the last 5 years, abnormal levels of eosinophils, haemoglobin (Hg), glycated haemoglobin (HbA1c), creatinine, thyroid-stimulating hormone (TSH), aspartate transaminase (ASAT), alanine transaminase (ALAT) and total bilirubin, positivity to serum protein electrophoresis and/or serum immunofixation, use of angiotensin-converting-enzyme (ACE) inhibitors, angiotensin (AT)-II antagonists, beta blockers, calcium (Ca) antagonists, antidiabetics, diuretics, anti-lipaemics and tranquilizers, clinical and aetiological classification of CP) were analysed by means of a data-mining algorithm that is able to compute and display the best connections between each pair of variables, taking into account other covariates in the system, as described elsewhere (6, 7).
Briefly, multiple logistic regression models were fitted by taking each time, sequentially, a variable as the predictor and the other as covariates. This process was reiterated until all variables in the model were processed. In addition, in order to avoid trivial associations, correlation among laboratory parameters was removed from the process. Finally, a matrix of normalized weights was produced from the regression coefficients. A mathematical filter, the maximum spanning tree (MST) (8), was then applied to the matrix of weights and a semantic connectivity map was generated. The MST selected positive associations ensuring to have normalized correlations in the interval [0, 1]. In addition, in order to avoid unstable associations, only connections with a p-value < 0.15 were considered by the algorithm. In the map, hubs of variables were detected, with straight lines showing the strongest associations, while spatial proximity between variables indicating patterns of direct correlations. The strength of associations can be interpreted as mild, moderate or strong for values < 0.6, 0.6–0.79 and ≥ 0.8, respectively (6). The analysis was carried out using MATLAB v.9.2 (MathWorks, Natick, MA, USA).
Patient demographics
Over a period of 6 years, 170 patients were hospitalized for evaluation of CP. The median duration of CP was 13.5 months (IQR 5–60 months). The frequency of patients with CP among all hospitalized patients in this period was 5%. The median age of all patients was 72 years (IQR 61–80 years). There was a predominance of females (58.2%). Based on the clinical presentation, 47.6% of all patients with CP had pruritus on diseased skin (CC-I). These patients had the following skin diseases: eczema, drug reaction, urticaria, bullous pemphigoid, psoriasis, scabies, mycosis fungoides, and Sézary syndrome. Furthermore, 17.6% of all patients had pruritus on non-diseased skin (CC-II) and 34.7% had pruritus with chronic scratch lesions (CC-III) (Table I). The aetiological classification based on the diagnoses and medical reports is listed in Table I.

Table I. Classification of chronic pruritus in 170 patients participating in the study according to clinical and aetiological categories
Semantic map analysis reveals sex-specific chronic pruritus patterns
On the semantic map showing the best associations among selected variables, the CC I–III are separated in different hubs (Fig. 1). CP associated with diseased skin (CC-I) and EC-I is linked to males. CC-II is not only associated with the diagnosis of cancer, but also links to abnormal bilirubin, ALAT and creatinine blood levels. Abnormal bilirubin levels are further linked to male sex, elevated HbA1c levels, indicating diabetic disposition and respective concomitant therapy in one hub, as well as to antihypertension drugs (primarily AT-II receptor antagonists) and diuretics. CC-III is associated with EC-V–VI as well as EC-III–IV, which is further connected to female sex, but also to AT-II receptor antagonists.
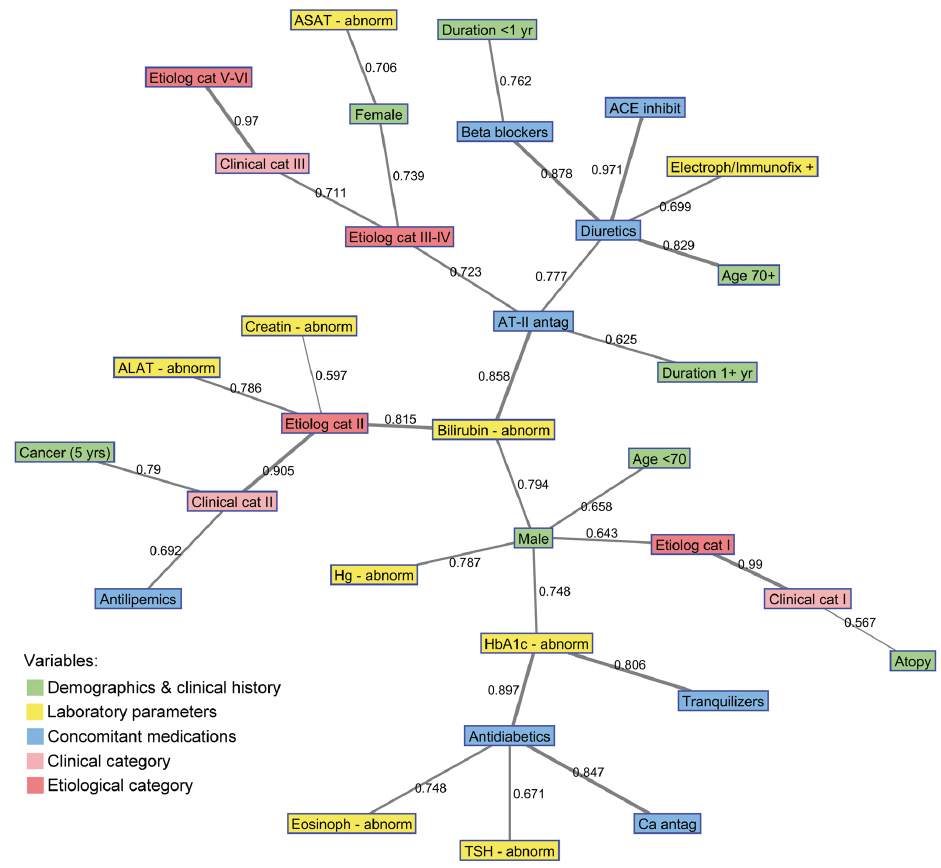
Fig. 1. Semantic map showing the best connections among selected variables in the study. The numbers on connecting lines indicate normalized correlations (between 0 and 1). The line thickness corresponds to the strength of association (thin line < 0.6; medium line 0.6–0.79; thick line ≥ 0.8). Abnorm: abnormal, ACE: angiotensin-converting-enzyme; ALAT: alanine transaminase; ASAT: aspartate transaminase; AT: angiotensin; Ca: calcium; Cat: category; Creatin: creatinine; Eosinoph: Electroph: electrophoresis, eosinophils; Etiolog: aetiological; HbA1c: glycated haemoglobin; Hg: haemoglobin; Immunofix: immunofixation; Inhibit: inhibitors; TSH: thyroid-stimulating hormone.
Diabetes and cancer are linked to pruritus on non-diseased skin and chronic scratch lesions
Comorbidities of patients with CP are reported in Table II. Hypertension (60%) was the most common comorbidity and equally represented in CC- I, II and III. The frequency of diabetes and cancer within the last 5 years was highest in patients with CC-II (Table II). Out of the patients with cancer diagnosis in the past 5 years, 13 patients had no active disease and in 5 patients the diagnosis of cancer was made by diagnostic work-up for CP. When patients were stratified based on aetiological causes, a high frequency of CLD was observed in EC-II, and in EC-III–IV and EC-V–VI. In CC-II and CC-III groups, the frequency of patients with CP with psychiatric comorbidities was higher compared with that of CC-I. Psychiatric comorbidities were identified in patients of all EC groups, with highest frequencies in EC-III–IV and EC-V–VI (Table II).
Among patients with CP on diseased skin, 51.9% presented with dry skin and 61.7% with eczematous lesions. Thus, papules and plaques were the most commonly observed primary skin lesions, and 90.6% of patients also had secondary lesions (Table SIII).
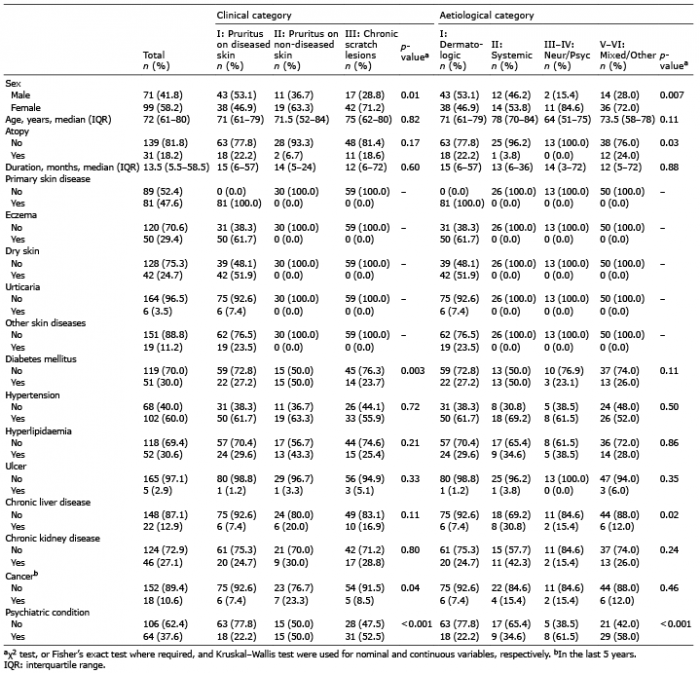
Table II. Demographics, comorbidities and clinical history of the study population, overall and in strata of clinical and aetiological categories of chronic pruritus
Eosinophil infiltration is a common finding in biopsies of patients with chronic pruritus
Light microscopy studies of 105 biopsy specimens were performed to either confirm or exclude a specific dermatosis that might be responsible for the CP. All histopathological slides were assessed by experienced dermatopathologists who performed a pattern analysis (9). The current study investigated whether the dermatopathological findings correlated with CP groups or aetiologies. It was notable that the presence of eosinophils in the skin was obvious in 72.2% of biopsies from patients with CP in CC-I and ≥ 50% in CC-II and III (Table SIV). The most common patterns described were spongiotic and eosinophilic patterns in CC-I, spongiotic and unspecific patterns in CC-II, and unspecific pattern in CC-III (Table SIV). As detected by direct immunofluorescence microscopy analysis, depositions of IgG, IgM, IgA and/or complement C3 were observed in samples obtained from all groups with > 60% in CC-I and III (Table SIV). Nevertheless, the rate of specific findings of direct immunofluorescence studies was very low. In fact, bullous pemphigoid was diagnosed in only 3 cases in patients with CP, based on characteristic immunopathological findings and clinical, histological and/or immunoserological criteria.
Blood eosinophil numbers, creatinine, and HbA1c levels show associations with chronic pruritus groups
A panel of laboratory parameters was then analysed. In CC-I and EC-I, the blood eosinophil levels were the high-est (Table SV). Correspondingly, the relative number of patients with elevated blood eosinophil levels was high-est in CC-I (Table SII). It is notable that, in patients with CP categorized as EC-II, creatinine levels were increased compared with all other EC groups, while HbA1c levels were highest in CC-II (Table SV). Significant differences in the frequency of patients with pathological laboratory results were found for urea, with the highest rate in EC-II. Infections were detected in 10/73 (12%), 4/81 (4.7%) and 1/78 (1.3%) of patients tested for hepatitis virus B and C, and HIV, respectively (Table SVI). In 32 patients with CP with suspected contact allergy, epicutaneous patch testing was performed that revealed at least one positive reaction in 13 patients (40.6%) (Table SVII).
Chronic pruritus and exposure to pharmacological therapies
It was further assessed whether CP was linked to concomitant medication in our patient cohort. No association was observed between clinical classification of CP and selected drug groups. When EC groups were analysed, a higher frequency of patients taking anti-arrhythmics, beta-blockers and AT-II antagonists was noticed in the EC-II and EC-III–IV groups (Table SVIII).
Whether and how CP and underlying diseases were treated was subsequently investigated. Before hospitalization, 76.5% of patients with CP applied a topical therapy (Table SIX). Notably, in one-third of the patients, CP and associated skin lesions were treated with systemic drugs, such as methotrexate, cyclosporine, retinoids and systemic corticosteroids. Systemic treatment was more frequently used in patients of CC-I compared with CC-II and III (p = 0.005). Oral antihistamines were used in 31.8% of patients with CP. Phototherapy was used in 21.2% of all patients with CP, mainly in those of CC-I and CC-II.
This study provides a comprehensive and detailed characterization of patients with CP referred to a tertiary university centre, by applying the criteria of CP classification using both semantic map and conventional statistical analyses. The semantic map, which shows the best associations between variables, clearly demonstrates an allocation of classification criteria and associated clinical and diagnostic variables in 5 distinct areas:
1. CC-I, EC-I;
2. CC-II, EC-II, abnormal liver/kidney parameters, history of cancer;
3. CC-III, EC-III–IV, EC-V–VI and females;
4. diabetes, antidiabetic therapy, and
5. anti-hypertensives and diuretics.
While hubs 1–3 show a direct link to the clinical presentation, hubs 4 and 5 largely lack such correlation.
A striking finding on the semantic map was the sex difference, which could be confirmed by classical statistical analysis. Females with CP more frequently presented with chronic scratch lesions and had concomitant neurological or psychiatric diseases, while CP on diseased skin predominated in males. These observations are in agreement with previous reports (10, 11). Sex differences in the perception of CP and its burden on quality of life have been reported. Following intracutaneous histamine challenge in patients with CP, females report more severe pruritus than males, despite the fact that the males have a larger wheal size (12). In females, CP affects quality of life more strongly than in males (10, 13).
In the cohort in the current study, CP owing to systemic diseases (EC-II), in particular chronic liver diseases, chronic kidney diseases, diabetes and diagnosis of cancer in the last 5 years, was common and mainly linked to primarily normal, non-inflamed skin (CC-II), as shown on the semantic map and by classical statistical analyses. It has been reported that, in patients with chronic liver diseases, over 40% have pruritus (14, 15). A viral infection seems to represent the most common cause of chronic cholestatic pruritus (16). In patients with chronic kidney disease, uraemic pruritus has been associated mainly with haemodialysis (17). In haemodialysis and in renal transplant patients CP is found in 62% and 32% of cases, respectively (18). Diabetes as comorbidity significantly appears to increase the risk of CP in geriatric patients in whom the prevalence of CP was 25% in one study (19). In elderly patients with diabetes mellitus, pruritus is thought to constitute the most common cutaneous symptom (20). Population-based studies have revealed an increased risk of malignancies, in particular haematological and bile duct malignancies, in patients with pruritus compared with those without pruritus (21–23). CP associated with malignancies presents on primarily non-inflamed skin with or without secondary scratch lesions (24). Nevertheless, there is increasing evidence that despite the different underlying clinical systemic diseases in patients with CP, the pathogenesis of itch is rather uniform, involving predominantly the IL-31/IL-31 receptor axis (25–28). Notably, we observed both tissue and blood eosinophilia frequently associated with CP. Eosinophils that are attracted and activated by IL-31, are also capable of releasing IL-31 and may thus contribute to itch, as in bullous pemphigoid (29, 30).
Pruritus is critically modulated by the psychological and emotional state of the patient, which can either cause or maintain pruritic symptoms (31). Among patients with CP referred by a dermatologist to a tertiary centre, 77.1% had at least 1 psychiatric/psychosomatic comorbidity (32). In our cohort, CP with severe chronic secondary scratch lesions (CC-III) was found to be associated with neurological/psychiatric diseases (EC-III/IV) and mixed/other diseases (EC-V/VI), as well as with female sex. This observation is in line with a previous report (10). Interestingly, pruritus on the head/neck area is most frequently reported by patients with CP who have neurological/psychiatric diseases. Notably, the link between hepatitis B and C viral infections and EC-III/IV and EC-V/VI could be traced back to chronic drug and alcohol abuse with or without concomitant internal diseases in these patients.
The association of CP with pharmacological treatments has been assumed, in particularly in elderly patients (33). However, in 1 investigation focusing on a geriatric population, no correlation between medication use and pruritus was found (19). A French case-control study reported a significant association between intake of calcium channel blockers and chronic eczematous eruption in elderly patients (34). In line with these findings, our study cohort showed a correlation of CP with hypertension and intake of anti-hypertensives, as well as diabetes and use of anti-diabetic drugs. Further investigation is needed to determine whether the association of AT II antagonist use with neurological/psychiatric diseases or abnormal blood bilirubin levels in patients with CP is clinically relevant or causative.
Study limitations
Limitations of this study include that it was a single-centre study, and that only patients hospitalized in a dermatological department have been analysed, while patients with CP cared for by other specialities were not included.
Conclusion
This study reveals a number of interesting associations between clinical presentations and aetiology, including pharmacological treatment, as well as sex differences in patients with CP by applying semantic map and classical statistical analyses. It should be noted that, despite these statistical correlations, there might be overlapping conditions in clinical practice. Therefore, the diagnostic work-up should consider: (i) the clinical presentation; (ii) a comprehensive patient history with focus on comorbidities and drug therapy; (iii) light microscopy and direct immunofluorescence studies of skin biopsy specimens, laboratory and imaging examinations (according to (ii)); and (iv) an evaluation on the psychological/psychosomatic status of patients with CP.