Nail psoriasis is a chronic, difficult-to-treat condition affecting almost half of patients with psoriasis. It is associated with considerable social stigma and impairment of patients’ quality of life. The aim of this study was to assess improvements in objective measures of nail psoriasis among patients from the long-term extension of the UNCOVER-3 study who received the interleukin-17A inhibitor ixekizumab and had either any degree of nail psoriasis (Nail Psoriasis Severity Index (NAPSI) >=1) or significant nail psoriasis (fingernail NAPSI ≥ 16 and ≥ 4 fingernails involved) at baseline. Efficacy outcomes reported through week 264 included the mean percentage improvements from baseline in NAPSI score and the proportion of patients achieving nail psoriasis resolution (NAPSI=0). In UNCOVER-3, 56.9% (219/385) of patients had nail psoriasis at baseline; of those, 61.2% (134/219) had significant nail psoriasis. At week 60, a total of 66.9% and 59.1% of patients with baseline nail psoriasis and significant baseline nail psoriasis, respectively, reported complete clearance of nail psoriasis, an effect which was sustained through week 264. This analysis demonstrates that continuous treatment with ixekizumab in adult patients with moderate-to-severe-psoriasis through 264 weeks was associated with improvements and clearance of fingernail psoriasis, irrespective of the severity of nail psoriasis at baseline.
Key words: ixekizumab; nails; psoriasis; Nail Psoriasis Severity Index; nail psoriasis.
Accepted Sep 19, 2022; Epub ahead of print Sep 19, 2022
Acta Derm Venereol 2022; 102: adv00787.
DOI: 10.2340/actadv.v102.2269
Corr: Alexander Egeberg, Department of Dermatology, Bispebjerg Hospital, University of Copenhagen, DK-2400 Copenhagen, Denmark. E-mail: alexander.egeberg@gmail.com
SIGNIFICANCE
Nail psoriasis is a chronic, difficult-to-treat, and often debilitating condition with a high disease burden and considerable impairment in quality of life. This study investigated the effect of continuous treatment with the therapeutic antibody ixekizumab on nail psoriasis. The results show that approximately 6 out of 10 patients achieved and maintained clearance of their fingernail psoriasis through 264 weeks, regardless of the severity of nail psoriasis when ixekizumab was commenced. These findings support the long-term efficacy of ixekizumab for the treatment of nail psoriasis and provide new insights into this burdensome and debilitating disease.
INTRODUCTION
Nail psoriasis (NP) is a chronic condition present in up to half of patients with psoriasis and is highly visible, interferes with manual dexterity, and can cause unpleasant sensations, such as pain (1). For these reasons, quality of life impairment among affected patients is often considerably more severe compared with involvement of other anatomical regions (2). Moreover, presence of nail changes is not only indicative of more severe psoriasis, but is also a predictor of the future development of psoriatic arthritis, thus supporting the need for early diagnosis and effective targeted treatment (3).
Ixekizumab (IXE) is a high-affinity monoclonal antibody that specifically inhibits interleukin (IL)-17A. It is approved for use in patients with psoriatic arthritis, axial spondyloarthritis, and in paediatric (≥ 6 years old) and adult patients with moderate-to-severe psoriasis. IXE has shown rapid onset of action with long-term efficacy in multiple phase III studies and IV trials (4, 5). UNCOVER-3, one of these studies, evaluated the long-term efficacy and safety of continuous treatment with IXE for up to 264 weeks (6). In UNCOVER-3, almost half of patients receiving IXE, as per label, demonstrated complete and sustained clearance of skin lesions (Psoriasis Area Severity Index, complete resolution of all lesions (100% improvement in Psoriasis Area and Severity Index (PASI 100)) by week 264, with more than 75% of patients with NP at baseline also achieving and maintaining complete clearance in their fingernail psoriasis (Nail Psoriasis Severity Index (NAPSI) of 0) (6).
This post hoc analysis from UNCOVER-3 describes the baseline characteristics of patients with NP, and reports improvements and complete clearance in NP composite and individual severity scores in patients randomly assigned to, and continuously receiving, IXE through week 264, who are stratified by baseline disease severity.
MATERIALS AND METHODS
Study design and evaluation of nail psoriasis
UNCOVER-3 was a phase III multicentre, double-blinded, placebo- and active comparator-controlled trial (NCT01646177); details of the study design and patient population have been reported previously (5). Nail involvement severity at baseline was assessed using NAPSI. Fingernails were divided into quadrants and the presence of any feature of nail matrix disease (i.e. pitting, crumbling, psoriatic leukonychia and red spots in the lunula) were scored 1 point per quadrant (maximum score 4). Fingernails were also evaluated for nail bed disease (i.e. onycholysis, splinter haemorrhages, subungual hyperkeratosis and oil-drop dyschromia) in the same manner as nail matrix features.
Patient subgroups
For this analysis, patients with moderate-to-severe psoriasis and concomitant fingernail psoriasis at baseline (defined as a NAPSI score ≥1; fingernail NAPSI range: 0–80) and receiving IXE (160 mg loading dose, followed by 80 mg Q2W until week 12 and, upon entering the 5-year long-term extension, Q4W thereafter) were followed through week 264. Data from patients who were dose-escalated to IXE Q2W after week 60 at the discretion of the investigator were excluded after switching. Patients at baseline who had any degree of NP, defined as NAPSI ≥1, were grouped as having “baseline” NP. From this group, patients who had moderate-to-severe NP, defined as fingernail NAPSI ≥16 and ≥4 fingernails involved, were sub-grouped as having “significant baseline” NP (7, 8).
Statistical analysis
Efficacy outcomes reported through week 264 included the mean percentage improvement from baseline in NAPSI score and the proportion of patients achieving NP resolution (NAPSI=0). Continuous outcomes were analysed using analysis of covariance (ANCOVA). For binary outcomes (e.g. the proportion of patients achieving NP resolution), confidence intervals were calculated using the normal approximation, without continuity correction. Efficacy data were summarized at each post-baseline visit through week 264, as observed, and by using 2 methods of missing data imputation, multiple imputation (MI), and modified non-responder imputation (mNRI) as described previously (6). Response rates using these 3 methods were summarized for categorical efficacy endpoints, and mean NAPSI percentage improvement (total and individual nail bed/nail matrix scores) was summarized using as observed and MI methods.
RESULTS
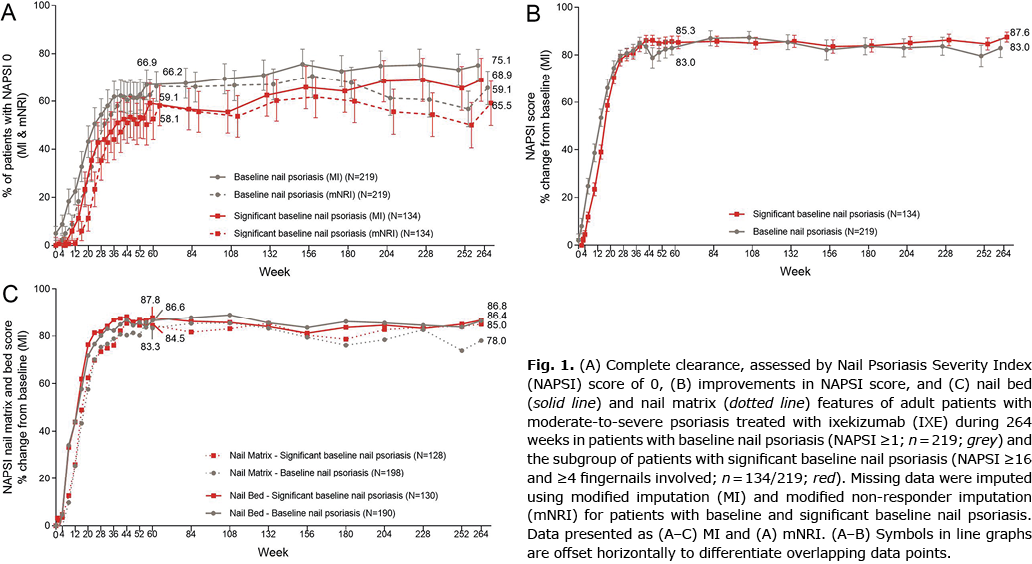
In UNCOVER-3, 56.9% (219/385) of patients had NP at baseline, of whom 61.2% (134/219) had significant NP (defined as fingernail NAPSI ≥ 16 and ≥ 4 fingernails involved). Patients with significant NP at baseline tended to be male, have a more severe psoriasis disease state (numerically higher PASI score), had more frequent involvement of palms and soles, and concomitant PsA compared with patients without NP (Table I). In addition, alcohol use and smoking were reported more frequently in patients with baseline NP compared with those without (Table I).
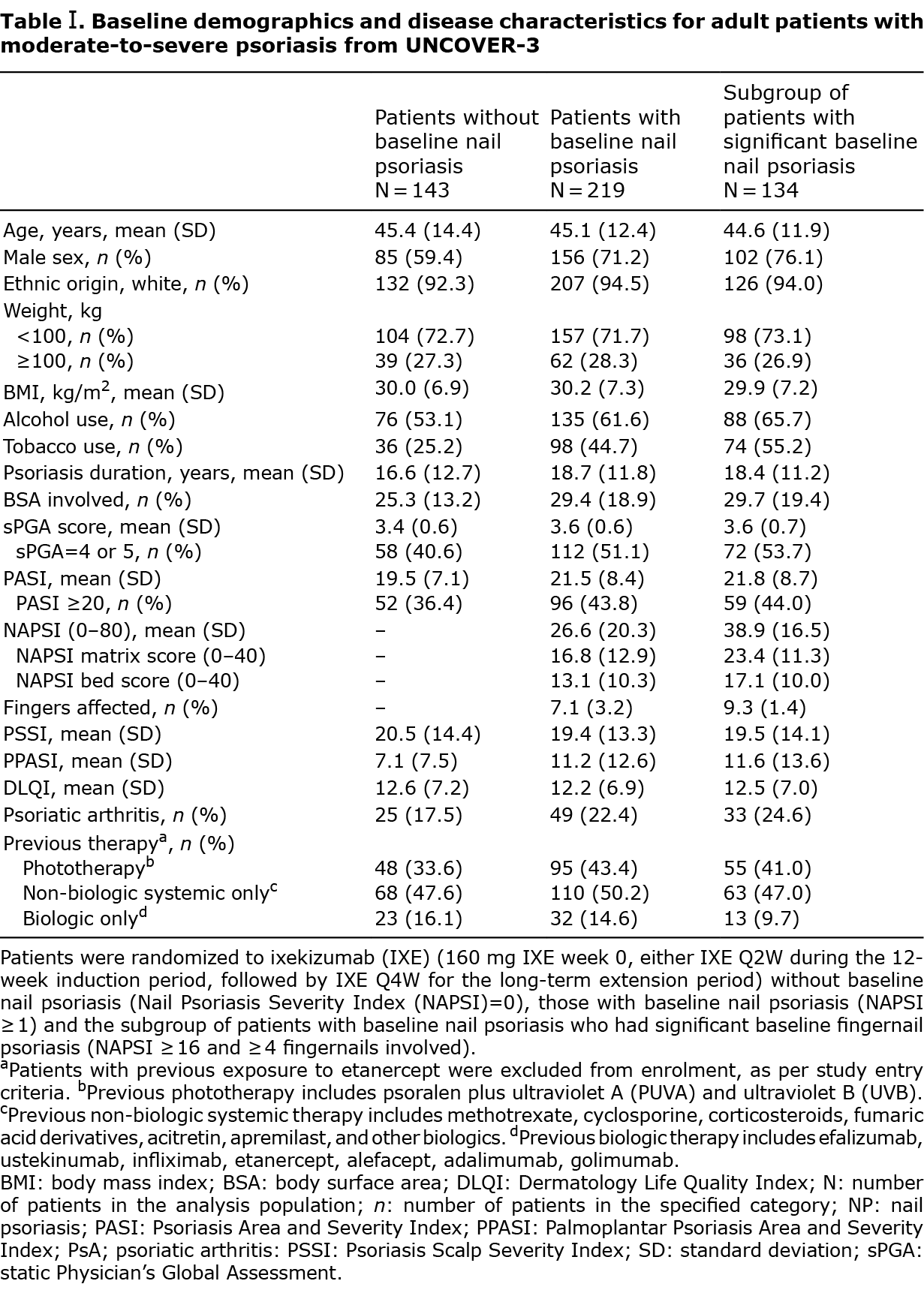
Complete clearance of NP was reported (MI) in 66.9% (66.2% mNRI) and 59.1% (58.1% mNRI) of patients with baseline NP and significant baseline NP, respectively, at week 60 and plateaued through week 264 (Fig. 1A (MI and mNRI); Fig. S1 (observed)).
The mean percentage improvements in total NAPSI score from baseline were comparable in IXE-treated patients across the NP groups, with a greater than 80% improvement by week 60, followed by consistent maintenance of efficacy through week 264 in patients with baseline and significant baseline NP (% NAPSI improvement (MI): 83.0% and 87.6%, respectively. Fig. 1B (MI); Fig. S2 (observed)).
Similar to previously reported results from IXE trials in paediatric patients with moderate-to-severe PsO (7), improvements in nail matrix scores (i.e. changes affecting primarily the nail plate) were marginally slower and slightly less pronounced at most time-points over the 264-week study period compared with nail bed scores and are probably related to the time required for the outgrowth of the nail plate (Fig. 1C (MI); Fig. S3 (observed)).
DISCUSSION
Management of NP is considered challenging, as the area is especially difficult to treat (1). In this post hoc analysis of patients with psoriasis with NP and significant NP at baseline, continuous treatment with IXE through 264 weeks was associated with improvements and clearance of fingernail psoriasis, irrespective of NP severity at baseline (6). Specifically, approximately half of all patients achieved complete clearance (NAPSI=0) by week 52, an effect which was sustained during 5 years. Similarly, approximately 80% improvement from baseline in NAPSI score (including for NAPSI bed and matrix scores) was observed until week 264. These data confirm and build on IXE’s long-term efficacy in patients with moderate-to-severe psoriasis.
IXE’s high efficacy in clearing NP has been demonstrated previously, with statistically higher responses rates in head-to-head studies vs guselkumab (at week 24) (9), and ustekinumab (through week 52) (8), as well as in recently published indirect comparisons vs other biologics or small molecules at weeks 24–26, consistently ranking IXE first (10). Clearance rates, arguably the most important treatment outcome for both patients and treating physicians (9), observed in those studies are comparable to the present results at corresponding time-points. In the network meta-analysis mentioned above, IXE showed the greatest complete resolution of NP at week 24‒26 (10). This study suggests that NP improvement and clearance in patients receiving IXE continues after week 26, with a maximum effect at around week 60 in both patients with baseline and patients with significant NP.
Improvements in nail matrix scores (i.e. changes affecting primarily the nail plate) were marginally slower and slightly less pronounced at most time-points over the 264-week study period compared with nail bed scores and are probably related to the time required for the outgrowth of the nail plate, a pattern comparable to what has been described for paediatric patients receiving IXE until week 48 (7).
Patients with NP are known to have more severe psoriasis and a higher frequency of comorbid psoriatic arthritis (11). In UNCOVER-3, too, patients with NP had higher PASI and body surface area scores, with even higher scores in patients with significant NP at baseline. Smokers are more likely to experience NP (12). Similarly, in UNCOVER-3, patients with NP and, even more so, patients with significant NP, were more frequently smokers and consumers of alcohol. Although the association between smoking and alcohol use and NP is not well-established, it is possible that smoking is another confounding factor present more frequently in patients with more severe disease (13).
NP typically precedes psoriatic arthritis and may be the first visible clinical sign indicative of psoriatic joint involvement (3). Because this association was also observed in the current study, targeted treatment of NP with biologics has recently been proposed as a potential means of lowering the risk of developing psoriatic arthritis in patients with chronic plaque psoriasis (3, 14). Further research is needed to determine whether this effect can be replicated in prospective studies, and whether differences exist among biologics based on their efficacy in improving and clearing NP.
Limitations of this post hoc analysis were the lack of a comparator arm and NP-specific quality of life (QoL) questionnaires, which precluded the assessment of the impact of NP on QoL, as well as the relatively small sample size and the lack of a comparison of mild with severe NP groups.
Overall, the results of this study support a clinically significant and 5-year sustained efficacy in NP observed in paediatric and adult populations with psoriasis and in adults with psoriatic arthritis treated with IXE (15), and provide new insights into this burdensome and debilitating disease.
ACKNOWLEDGEMENTS
Eli Lilly and Company (Indianapolis, IN, USA) sponsored this study and the medical writing (Gerard Sheehan, PhD, and Edel Hughes, PhD) for this paper. The authors thank Missy Mc-Kean-Matthews for statistical review of the article.
This study was sponsored by Eli Lilly and Company (Indianapolis, IN, USA).
Study protocols and informed consent forms were approved by an investigational review board at each site, and all patients signed informed consent before undergoing study-related procedures.
Conflicts of interest: AE received research funding from AbbVie, Danish National Psoriasis Foundation, Eli Lilly and Company, Janssen, Kgl. Hofbundtmager Aage Bangs Foundation, Novartis, Pfizer and Simon Spies Foundation, received consulting fees from AbbVie, Almirall, Bristol-Myers Squibb, Dermavant, Eli Lilly and Company, Galderma, Janssen, LEO Pharma, Mylan, Novartis, Pfizer, Samsung Bioepis Co., Ltd, UCB and Union Therapeutics, payment for honoraria or speaking from AbbVie, Almirall, Bristol-Myers Squibb, Dermavant, Eli Lilly and Company, Galderma, Janssen, LEO Pharma, Mylan, Novartis, Pfizer, Samsung Bioepis Co., Ltd, and UCB, support for congress travel from AbbVie, and participation on a data safety monitoring or advisory board from Samsung Bioepis Co., Ltd. LEK received research grants from Pfizer, AbbVie, UCB, Gilead, Biogen, Novartis, Eli Lilly and Company, and Janssen Pharmaceuticals, has received consulting fees from Pfizer, AbbVie, Amgen, UCB, Gilead, Biogen, BMS, MSD, Novartis, Eli Lilly and Company, and Janssen Pharmaceuticals, and fees for speaking from Pfizer, AbbVie, Amgen, UCB, Gilead, Biogen, BMS, MSD, Novartis, Eli Lilly and Company, and Janssen Pharmaceuticals. RV has received grants or research funding from AbbVie, Amgen, Arcutis, Bausch Health, BMS, Celgene, Centocor, Dermira, Dermavant, Galderma, GSK, Innovaderm, Janssen, Leo, Eli Lilly and Company, Meiji, Takeda, Novartis, Merck, Pfizer, Regeneron, UCB consulting fees from AbbVie, Actelion, Amgen, Aralez, Arcutis, Bausch-Health, Boehringer Ingelheim, BMS, Celgene, Cipher, Janssen, Galderma, GSK, Kabi-Care, Leo, Eli Lilly and Company, Merck, Novartis, Palladin, Pfizer, Sandoz, SUN, UCB, Viatris-Mylan, fees for speakers bureau or honoraria from AbbVie, Actelion, Amgen, Bausch-Health, Celgene, Cipher, Janssen, Galderma, GSK, Leo, Eli Lilly and Company, Merck, Novartis, Pfizer, UCB, and support for meetings and/or travel from AbbVie, Amgen, Bausch Health, Celgene, Galderma, Janssen, Leo, Lilly, Novartis, UCB. SZ has served as an advisory board member or received fees and speaker’s honoraria from AbbVie, Biogen, LEO Pharma, and Novartis. CEB was an independent contractor working for Eli Lilly and Company at the time of writing GG, ER, CS are employees and shareholders of Eli Lilly and Company.
REFERENCES
- Haneke E. Nail psoriasis: clinical features, pathogenesis, differential diagnoses, and management. Psoriasis (Auckl) 2017; 7: 51–63.
- Blome C, Augustin M, Klein TM. Nail psoriasis and quality-of-life measurement in clinical trials: call for the use of nail-specific instruments. Am J Clin Dermatol 2021; 22: 747–755.
- Gisondi P, Bellinato F, Targher G, Idolazzi L, Girolomoni G. Biological disease-modifying antirheumatic drugs may mitigate the risk of psoriatic arthritis in patients with chronic plaque psoriasis. Ann Rheum Dis 2022; 81: 68–73.
- Paller AS, Seyger MMB, Alejandro Magarinos G, Bagel J, Pinter A, Cather J, et al. Efficacy and safety of ixekizumab in a phase III, randomized, double-blind, placebo-controlled study in paediatric patients with moderate-to-severe plaque psoriasis (IXORA-PEDS). Br J Dermatol 2020; 183: 231–241.
- Craig S, Warren RB. Ixekizumab for the treatment of psoriasis: up-to-date. Expert Opin Biol Ther 2020; 20: 549–557.
- Blauvelt A, Lebwohl MG, Mabuchi T, Leung A, Garrelts A, Crane H, et al. Long-term efficacy and safety of ixekizumab: A 5-year analysis of the UNCOVER-3 randomized controlled trial. J Am Acad Dermatol 2021; 85: 360–368.
- Seyger MMB, Reich A, El Baou C, Schuster C, Riedl E, Paller AS. Efficacy of ixekizumab on nail psoriasis in paediatric patients with moderate-to-severe psoriasis: a post hoc analysis from IXORA-PEDS. J Eur Acad Dermatol Venereol 2021; 35: e911–e913.
- Wasel N, Thaci D, French LE, Conrad C, Dutronc Y, Gallo G, et al. Ixekizumab and ustekinumab efficacy in nail psoriasis in patients with moderate-to-severe psoriasis: 52-week results from a phase 3, head-to-head study (IXORA-S). Dermatol Ther (Heidelb) 2020; 10: 663–670.
- Blauvelt A, Leonardi C, Elewski B, Crowley JJ, Guenther LC, Gooderham M, et al. A head-to-head comparison of ixekizumab vs. guselkumab in patients with moderate-to-severe plaque psoriasis: 24-week efficacy and safety results from a randomized, double-blinded trial. Br J Dermatol 2021; 184: 1047–1058.
- Reich K, Conrad C, Kristensen LE, Smith SD, Puig L, Rich P, et al. Network meta-analysis comparing the efficacy of biologic treatments for achieving complete resolution of nail psoriasis. J Dermatolog Treat 2022; 33: 1652–1660.
- Augustin M, Reich K, Blome C, Schafer I, Laass A, Radtke MA. Nail psoriasis in Germany: epidemiology and burden of disease. Br J Dermatol 2010; 163: 580–585.
- Temiz SA, Ozer I, Ataseven A, Dursun R, Uyar M. The effect of smoking on the psoriasis: Is it related to nail involvement? Dermatol Ther 2020; 33: e13960.
- Armstrong AW, Armstrong EJ, Fuller EN, Sockolov ME, Voyles SV. Smoking and pathogenesis of psoriasis: a review of oxidative, inflammatory and genetic mechanisms. Br J Dermatol 2011; 165: 1162–1168.
- Acosta Felquer ML, LoGiudice L, Galimberti ML, Rosa J, Mazzuoccolo L, Soriano ER. Treating the skin with biologics in patients with psoriasis decreases the incidence of psoriatic arthritis. Ann Rheum Dis 2022; 81: 74–79.
- Reich K, Kristensen LE, Smith SD, Rich P, Sapin C, Liu-Leage S, et al. Efficacy and safety of ixekizumab versus adalimumab in biologic-naïve patients with active psoriatic arthritis and moderate-to-severe psoriasis: 52-week results from the randomized SPIRIT-H2H trial. Dermatol Pract Concept 2022; 12: e2022104.