Multiple Progressive Necrotic Lesions in a Young Man: A Quiz
Yurong Li1–4, Ping Tu1–4, Yang Wang1–4 and Jingru Sun1–4*
1Department of Dermatology and Venereology, Peking University First Hospital, No.8 Xishiku Street, Xi Cheng District, Beijing 100034, 2National Clinical Research Center for Skin and Immune Diseases, 3Beijing Key Laboratory of Molecular Diagnosis on Dermatoses, and 4NMPA Key Laboratory for Quality Control and Evaluation of Cosmetics, Beijing, China. *E-mail: sjr12315@126.com
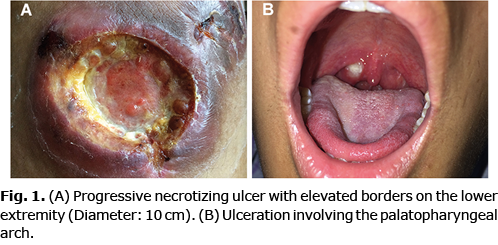
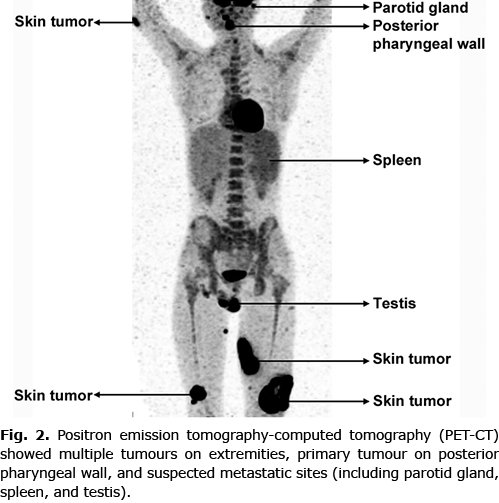
An 18-year-old man presented with a history of multiple progressive necrotizing lesions on his lower extremities, and intermittent fever. Seven months previously, an erythematous painful lesion had developed on his left lower leg, which eventually evolved into a tumour and subsequently ulcerated. During this time, multiple similar progressive ulcerating lesions appeared symmetrically on both lower extremities. The patient also had intermittent fever with a maximum temperature of 40°C, accompanied by malaise and drenching night sweats. He denied nasal obstruction or epistaxis. No exposure to pesticides or chemical solvents was recalled. His family members did not have any Epstein-Barr virus related diseases. Physical examination revealed multiple ill-defined indurated ulcers with elevated borders on his lower extremities, of which the largest was 10 cm in diameter (Fig. 1A). Notably, an ulcer was detected on his palatopharyngeal arch (Fig. 1B). Laboratory workup showed increased levels of lactate dehydrogenase (LDH 598 IU/l, normal range: 100–240 IU/l), NK cell absolute count (2,851.25/µl, normal range: 84.0–724.0/µl) and plasma Epstein-Barr virus-DNA (EBV-DNA 9.93×103 copies/ml, normal range: < 500 copies/ml). Bone marrow aspiration showed marked hyperplasia of bone marrow with scattered haemophagocytic cells. Positron emission tomography-computed tomography (PET-CT) was performed, demonstrating multiple sites with suspected malignancy involvement, including skin of the extremities, posterior pharyngeal wall, parotid gland, spleen, and testis (Fig. 2).
What’s your diagnosis? See next page for answer.
Multiple Progressive Necrotic Lesions in a Young Man: A Commentary
Acta Derm Venereol 2022; 102: adv00794.
DOI: 10.2340/actadv.v102.2308
Diagnosis: Extranodal NK/T-cell lymphoma, nasal type
Extranodal NK/T-cell lymphoma, nasal type (ENKTL-NT) is a rare aggressive non-Hodgkin’s lymphoma originating from NK cells or cytotoxic T cells, which often occurs in East Asia and Latin America. It is first included in the WHO classification of lymphomas in 2001 and maintained since then. Males and adults are more usually affected than females and children (1).
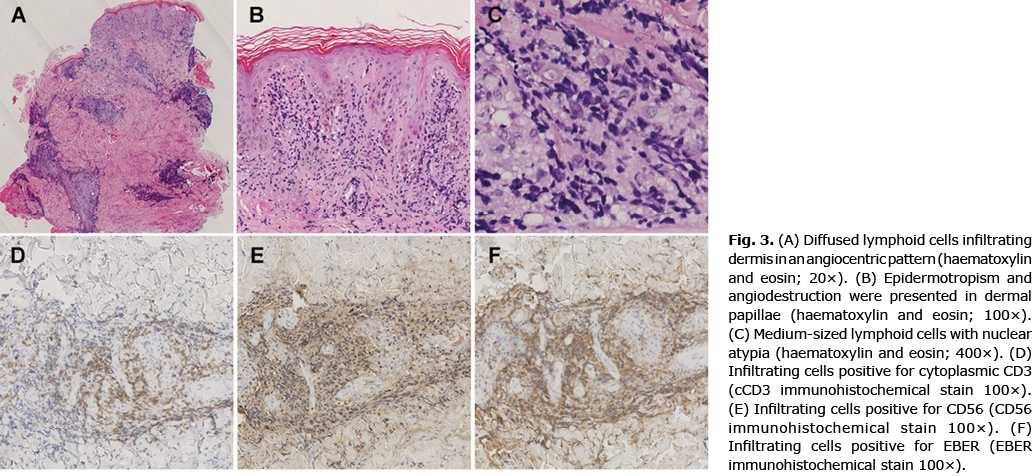
Skin biopsy of the largest lesion showed diffused atypical lymphoid cells infiltrating dermis in an angiocentric pattern (Fig. 3A). Epidermotropism and angiodestruction were also presented (Fig. 3B–C, Fig. S1). The infiltrating cells were positive for CD2, cytoplasmic CD3 (Fig. 3D), CD56 (Fig. 3E), EBV encoded RNA (EBER, Fig. 3F), granzyme B, T cell intracellular antigen-1 (TIA-1) and T-bet, with almost no expression of CD20, CD30, and CD123. T-cell receptor (TCR) analysis of his biopsy was negative for rearrangements of TCRB, TCRG, and TCRD.
The initial symptom of more than 80% of patients was nasal obstruction and ulcerative necrotic lesions affecting the nasal skin and mucosa. However, in approximately 20% of patients, lymphoma can arise in the other skin areas, gastrointestinal tract and testis, manifesting as progressive ulcerated lesions (2). Typical skin manifestations often begin as multiple erythematous plaques or masses on the trunk or extremities, which gradually develop into ulcers as the disease progresses. Some patients may also have B symptoms, such as fever, night sweats and weight loss (3). Lymph node involvement may occur. Bone marrow and peripheral blood can become involved in aggressive cases (4).
As many diseases could present as multiple necrotic lesions, making a correct diagnosis of ENKTL-NT is often challenging. Histopathologically, ENKTL-NT is characterized by atypical polymorphic lymphocytes showing angiocentric infiltrating pattern, sometimes accompanied by vascular destruction and necrosis. Due to the presence of numerous necrotic backgrounds and mixtures of inflammatory cells, immunohistochemical stains are needed for diagnosis. Since the neoplastic cells are derived from NK cells or T cells, they are positive for CD2, cytoplasmic CD3, CD56, T-bet, and cytotoxic molecules (granzyme B, perforin, TIA1). However, T-cell surface markers, such as CD3, CD4, and CD8, may be negative in ENKTL-NT derived from NK cells (5, 6). Most of ENKTL-NT cases have an NK phenotype without clonal TCR rearrangements, whereas T-cell-derived cases could be positive for clonal TCR rearrangements (7).
According to the pathogenesis related to EBV infection, the elevated level of EBV in plasma and the positive result of EBER by in situ hybridization or immunohistochemistry are crucial to the diagnosis of ENKTL-NT (8). Chronic active EBV infection may escape immune surveillance, leading to a risk of its occurrence. Subsequent genetic abnormalities in infected cells are needed to promote tumourigenesis (9). Moreover, periodic measurement of serum levels of EBV DNAs is useful for monitoring tumour progression and is considered to be associated with a higher clinical stage and poor prognosis (10). In addition, examinations, such as peripheral blood flow cytometry, bone marrow biopsy and PET-CT, are necessary to evaluate the stage and extent of the disease.
Therapy for ENKTL-NT has limited effectiveness. Radiation therapy is considered as recommended first-line therapy for localized tumours, while chemotherapy and hematopoietic stem cell transplantation are recommended for systemic conditions (11). Patients with only skin lesions have a better prognosis than patients who have both skin and other organ involvement. Rare cases with complications of haemophagocytic lymphohistiocytosis have an extremely poor prognosis. Fortunately, the current patient did not complicate with haemophagocytic syndrome. A recent study has found that programmed cell death-1 (PD-1) and programmed cell death-ligand-1 (PD-L1) blockade might be a promising strategy for patients with relapsed, refractory ENKTL-NT (12). As a considerable proportion of ENKTL-NT cases express CD30, CD38, CD52, and CD74, antibodies against these targets have also been applied in limited ENKTL-NT cases. Furthermore, novel therapeutic strategies, including employing cytotoxic-T cells against EBV latent membrane protein, inhibition of the JAK/STAT and NF-κB signalling pathways have been recommended for some relapsed or refractory ENKTL-NT cases, although these need further investigation (1).
In conclusion, when progressive ulcerating lesions dominate the clinical picture, it is important to be alert for ENKTL-NT as a differential diagnosis.
ACKNOWLEDGEMENTS
This study was supported by Beijing Natural Science Foundation (7214260) of Jingru Sun.
Patient consent has been obtained.
The authors have no conflicts of interest to declare.
REFERENCES
- Sanchez-Romero C, Bologna-Molina R, Paes de Almeida O, Santos-Silva AR, Prado-Ribeiro AC, Brandao TB, et al. Extranodal NK/T cell lymphoma, nasal type: an updated overview. Crit Rev Oncol/Hematol 2021; 159: 103237.
- Goodlad JR. Epstein-Barr virus-associated lymphoproliferative disorders in the skin. Surg Pathol Clin 2017; 10: 429–453.
- Jhuang J-Y, Chang S-T, Weng S-F, Pan S-T, Chu P-Y, Hsieh P-P, et al. Extranodal natural killer/T-cell lymphoma, nasal type in Taiwan: a relatively higher frequency of T-cell lineage and poor survival for extranasal tumors. Hum Pathol 2015; 46: 313–321.
- Tse E, Kwong Y-L. Diagnosis and management of extranodal NK/T cell lymphoma nasal type. Expert Rev Hematol 2016; 9: 861–871.
- Pongpruttipan T, Sukpanichnant S, Assanasen T, Wannakrairot P, Boonsakan P, Kanoksil W, et al. Extranodal NK/T-cell lymphoma, nasal type, includes cases of natural killer cell and αβ, γδ, and αβ/γδ T-cell origin: a comprehensive clinicopathologic and phenotypic study. Am J Surg Pathol 2012; 36: 481–499.
- Li S, Feng X, Li T, Zhang S, Zuo Z, Lin P, et al. Extranodal NK/T-cell lymphoma, nasal type: a report of 73 cases at MD Anderson Cancer Center. Am J Surg Pathol 2013; 37: 14–23.
- Geller S, Myskowski PL, Pulitzer M. NK/T-cell lymphoma, nasal type, gammadelta T-cell lymphoma, and CD8-positive epidermotropic T-cell lymphoma-clinical and histopathologic features, differential diagnosis, and treatment. Semin Cutan Med Surg 2018; 37: 30–38.
- Tse E, Kwong Y-L. The diagnosis and management of NK/T-cell lymphomas. J Hematol Oncol 2017; 10: 85.
- Sánchez-Romero C, Bologna-Molina R, Paes de Almeida O, Santos-Silva AR, Prado-Ribeiro AC, Brandão TB, et al. Extranodal NK/T cell lymphoma, nasal type: an updated overview. Crit Rev Oncol Hematol 2021; 159: 103237.
- Takahara M, Kumai T, Kishibe K, Nagato T, Harabuchi Y. Extranodal NK/T-cell lymphoma, nasal type: genetic, biologic, and clinical aspects with a central focus on Epstein-Barr virus relation. Microorganisms 2021; 9: 1381.
- Yamaguchi M, Suzuki R, Oguchi M. Advances in the treatment of extranodal NK/T-cell lymphoma, nasal type. Blood 2018; 131: 2528–2540.
- Yamaguchi M, Miyazaki K. Current treatment approaches for NK/T-cell lymphoma. J Clin Exp Hematop 2017; 57: 98–108.


