Nail dermoscopy (onychoscopy) is a valuable diagnostic tool for evaluating diseases in the nail apparatus. It is non-invasive, allowing clinicians to prioritize particular nails for biopsy. Thus, it can improve diagnostic accuracy and expedite treatment. Evaluating inflammatory nail disorders using onychoscopy is a relatively new approach to clinical assessment and has the potential to augment clinical care. This review highlights key dermoscopic features of major inflammatory nail disorders, including trachyonychia, nail psoriasis, nail lichen planus, onychotillomania, nail lichen striatus and allergic contact dermatitis due to artificial nails. It also illustrates their management and differential diagnoses, including onychomycosis, onycholysis, nail dystrophy due to systemic amyloidosis and malignant nail tumours. Limitations of this review included the low amount of literature on this topic and non-standardized terminology used among researchers. As onychoscopy is a relatively new technique, further studies and standardization of terminology are warranted to consolidate the role of dermoscopy in evaluating inflammatory nail disorders.
Key words: trachyonychia; psoriasis; lichen planus; lichen striatus; onychotillomania; allergic contact dermatitis.
Accepted Sep 2, 2021; Epub ahead of print Sep 7, 2021
Acta Derm Venereol 2021; 101: adv00548.
doi: 10.2340/00015555-3917
Corr: Je-Ho Mun, Department of Dermatology, Seoul National University College of Medicine, Seoul, Republic of Korea. E-mail: jehomun@gmail.com
SIGNIFICANCE
Nail dermoscopy (onychoscopy) is a valuable diagnostic aid for evaluating diseases in the nail apparatus. It is non-invasive, allowing clinicians to prioritize particular nails for biopsy. Thus, it can improve diagnostic accuracy and expedite treatment. Evaluating inflammatory nail disorders using onychoscopy is a relatively new approach to clinical assessment and has the potential to augment clinical care. This review highlights key dermoscopic features of major inflammatory nail disorders. It also illustrates their management and differential diagnoses, including onychomycosis, onycholysis, nail dystrophy due to systemic amyloidosis, and malignant nail tumours.
INTRODUCTION
Nail dermoscopy (onychoscopy) is a bedside tool used to evaluate nail diseases. Commonly used to assess melanonychia and potential tumours, onychoscopy is increasingly recognized as a valuable examination tool for inflammatory nail disorders. Appreciating key dermoscopic features of nail disorders can improve diagnostic accuracy, guide prognosis, minimize the need for unnecessary biopsies, and optimize treatment. This review outlines characteristic dermoscopic features of major inflammatory nail disorders: trachyonychia, nail psoriasis, nail lichen planus, onychotillomania, nail lichen striatus and allergic contact dermatitis due to artificial nails. Dermoscopic features of mimics of inflammatory nail disorders are also discussed. PubMed was searched for studies published to 30 May 2020 in English, using the following key words: dermoscopy or its synonyms (dermatoscopy, videodermoscopy, onychoscopy), trachyonychia, psoriasis, lichen planus, onychotillomania, lichen striatus, contact dermatitis or inflammatory nail disorders. Relevant articles from this search, which discuss inflammatory nail disorders and their dermoscopic patterns, were included in this review.
TRACHYONYCHIA
Trachyonychia is an inflammatory nail disease characterized by a rough and brittle nail surface (Fig. 1) (1–3). Trachyonychia is usually primary and idiopathic (4). However, it can arise secondary to dermatological conditions, such as alopecia areata, psoriasis, lichen planus, ichthyosis vulgaris, vitiligo and atopic dermatitis (4–6). “Twenty-nail dystrophy” refers to the acquired idiopathic form involving all 20 nails, although this term is controversial as trachyonychia can affect any number of nails (1, 3, 4). Trachyonychia can be classified into opaque and shiny subtypes, with the former associated with greater inflammation (3, 7). In opaque trachyonychia, nails are “sandpaper-like” with longitudinal ridging, onychoschizia (irregular horizontal splitting of the distal plate layers) and hyperkeratotic cuticles (1, 4). In shiny trachyonychia, nails are opalescent with small geometric pits reflecting light and forming longitudinal ridges (1, 4).

Onychoscopy can assist in the diagnosis of trachyonychia. In a recent onychoscopic analysis of 30 trachyonychia cases, distinctive changes were reported in the nail plate, cuticle, and periungual skin (Fig. 1). These included scaling (100%), longitudinal ridging (93%), involvement of the proximal nail plate (93%), involvement of > 50% of the proximal nail plate width (90%), splinter haemorrhages (70%), pitting (33%), onychoschizia (33%) and longitudinal melanonychia (brown-black pigmented bands on nail plate) (7%) (8). Lunulae were mostly opaque (43%) or red (40%). Thickened and ragged cuticles (87%) and periungual scales (63%) were also observed (Table I).

Trachonychia can have a long-lasting course in adults, and its cosmetic appearance can reduce patients’ quality of life (9). Therefore, anti-inflammatory therapies are recommended. Topical corticosteroids, urea-containing ointments and calcipotriol/betamethasone dipropionate are typical first-line therapies (3, 4, 10). Other treatment options include intralesional steroid injections and systemic treatments, such as corticosteroids, acitretin and cyclosporine. Systemic therapies can be used for patients with severe or refractory trachyonychia.
NAIL PSORIASIS
Psoriasis is an immune-mediated inflammatory dermatosis. Nail psoriasis affects up to 50% of patients with chronic plaque psoriasis (11, 12). It is functionally debilitating, reducing patients’ quality of life (11, 13). It is associated with greater mean Psoriasis Area and Severity Index (PASI) scores and disease duration, and is an independent predictive factor for development of psoriatic arthritis (13–17).
Onychoscopy is useful in detecting subclinical nail psoriasis (Fig. 2) (18). Pitting, distal onycholysis (separation of nail plate from underlying nail bed) and splinter haemorrhages are key dermoscopic features of nail psoriasis (11, 18–21). Signs of nail matrix involvement include pitting, rough and brittle nails and deep transverse grooves (11, 18, 21, 22). Signs of nail bed and hyponychial involvement include distal onycholysis with a yellow-orange and dented proximal margin, splinter haemorrhages, oil drop/salmon patch, subungual hyperkeratosis, red/black haemorrhagic dots and hyponychial capillaries, which are dilated, tortuous, elongated and irregularly distributed (18, 21–25). Crumbling of the emerging nail plate and dilated hyponychial capillaries suggest severe nail disease (11, 26).

Onychoscopy may be useful for monitoring treatment response, although research in this area is currently limited. Iorizzo et al. (23) reported that there were fewer visible hyponychial capillaries after a 3-month course of calcipotriol ointment twice a day. Hashimoto et al. (24) noted that resolution of diffuse scaling of the nail plate, transverse step-like notches, thickened white-yellow nail plates and splinter haemorrhages were associated with improvements in patients’ PASI scores after biologic treatment.
Treatment of nail psoriasis depends on the degree of nail involvement. An expert consensus recommends intralesional steroid injections for nail matrix psoriasis, and topical steroids alone or with vitamin D analogues for nail bed psoriasis (27). Systemic therapies are considered when more than 3 nails are involved, the patient has symptomatic psoriatic arthritis, or the disease significantly impacts patients’ quality of life (27). Such therapies include acitretin, methotrexate, cyclosporin, small molecules and biologics, such as infliximab, adalimumab, golimumab, ustekinumab, ixekizumab and tofacitinib. To maximize the effectiveness of treatment, nail trauma minimization is important.
NAIL LICHEN PLANUS
Lichen planus (LP) is a chronic inflammatory dermatosis characterized by violaceous flat-topped polygonal papules surmounted by Wickham’s striae (whitish lines) variably involving mucocutaneous surfaces, scalp and nails (28). Nail involvement occurs in 10% of patients (29, 30). Early identification is crucial, as nail LP can cause extensive damage to the nail matrix leading to anonychia and proximal pterygium (21, 29, 30).
Onychoscopy can help recognize early changes of nail LP (Fig. 3). Features of nail matrix involvement include rough and brittle nails, pitting, red or mottled lunula, onychorrhexis (vertical nail plate ridges), longitudinal streaks and longitudinal splitting (20–22, 29). Features reflecting nail bed involvement include nail fragmentation, chromonychia (discolouration of nail plate or subungual tissue), splinter haemorrhages, onycholysis, subungual hyperkeratosis, and longitudinal grooves converging to the centre of the nail apparatus (20–22, 29). Longitudinal linear dyschromic (alternating blue, brown or black) bands have also been reported in nail LP (31).
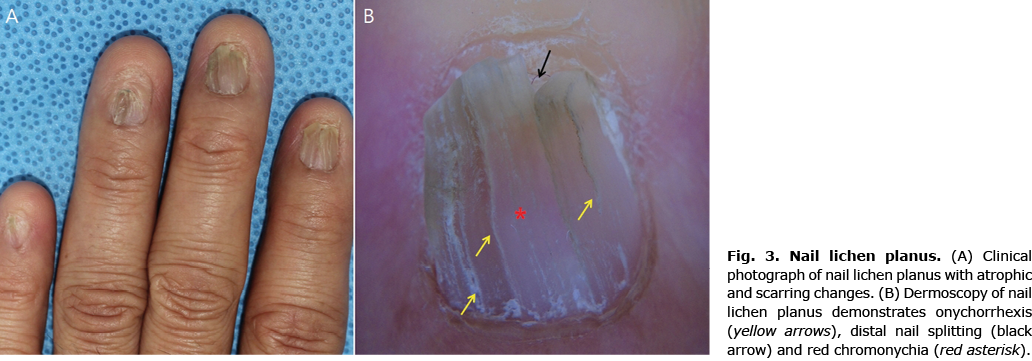
As nail LP causes irreversible scarring and nail loss, prompt treatment is warranted (32). To date, there are no evidence-based management guidelines for nail LP. An expert consensus recommends intralesional and intramuscular steroid injections (triamcinolone acetonide) as first-line treatments (33). Oral retinoids and immunosuppressants, such as azathioporine, cyclosporine, and mycophenolate mofetil, may be employed as second- and third-line choices.
ONYCHOTILLOMANIA
In onychotillomania, onychodystrophy results from repetitive self-induced trauma (34). It sometimes overlaps with other behavioural disorders of the nail, including onychophagia and habit tic deformity (35). Although onychotillomania is a psychodermatological condition associated with obsessive-compulsive disorder, depression, and specific phobias, it results in inflammatory changes in the nail unit caused by repetitive manipulation. Onychotillomania is not always a straightforward diagnosis. The clinical and histopathological features are variable and non-specific, and patients often deny their self-destructive behaviours (35, 36).
In a recent study investigating 36 onychotillomania cases, Maddy et al. found that dermoscopic features of onychotillomania included scales in the nail bed, nail folds and hyponychium (94.4%), absence of nail plate (83.3%), wavy lines (69.4%), obliquely oriented haemorrhages (63.9%), crusts (61.1%), nail bed pigmentation (47.2%), speckled dots (38.9%) and melanonychia (11.1%) (Fig. 4) (34). Identifying such dermoscopic features on a background of psychopathology raises suspicion of onychotillomania.

Building patients’ trust and encouraging them to recognize that their behaviours are important, as clinicians should couple psychotherapy with physical treatment to prevent further injury (35). A recent meta-analysis analysing treatments for skin-picking disorders reported that behavioural modification therapy was significantly more beneficial than inactive control conditions (37). However, there is insufficient evidence on psychopharmacological agents providing significant benefit compared with placebo (37). If there are overt inflammatory changes in the nails, barrier creams with or without topical steroids can facilitate recovery.
NAIL LICHEN STRIATUS
Cutaneous lichen striatus (LS) is an inflammatory linear dermatosis with pink or red flat-topped papules arranged along Blaschko’s lines (38). In LS, acquired stimuli, such as infection and trauma, trigger loss of immunotolerance to embryologically abnormal clones, causing a T-cell mediated inflammatory response (39). Nail LS is rare, but important, to recognize, as it can cause irreversible nail damage in some cases (40, 41).
Few studies have described the dermoscopic features of nail LS. Reported nail plate changes include fissuring, longitudinal ridging, onychoschizia, and distal nail plate splitting (Fig. 5) (42–44). Red spots or bands in the lunula and subungual hyperkeratosis may also be observed (42, 43). Nail changes are accompanied by adjacent skin changes, such as brownish keratotic nutmeg structures with red dots surrounded by a pale halo, or brownish to greyish granular pigmentation with dotted vessels and white scales (42, 44).

As nail LS usually resolves spontaneously, most cases are managed conservatively. Treatment is reserved for persistent or severe inflammatory cases. Potent topical steroids or calcineurin inhibitors are considered first-line, while intralesional steroid injections can be trialled in refractory cases (45).
ALLERGIC CONTACT DERMATITIS DUE TO ARTIFICIAL NAILS
Artificial nails contain acrylates and methacrylates, whose polymers trigger allergic contact dermatitis (ACD) (46). ACD due to artificial nails usually affects fingers, hands and/or wrists (88.9%), but also the face (36.8%), mainly the eyelids, lips and cheeks (46).
ACD due to artificial nails is a pertinent dermatological and occupational health issue, with its incidence rising in nail technicians and consumers (46, 47). In 2017 a multi-centre study reported that 136 out of 202 positive reactions to acrylates in patch tests were associated with nail acrylates (0.75% of all patients) (46).
Onychodystrophy in ACD due to artificial nails is characterized primarily by onycholysis and subungual hyperkeratosis (48, 49). Nail changes can resemble nail psoriasis, but the 2 conditions can be differentiated on onychoscopy (50). Key dermoscopic features of ACD due to artificial nails include onycholysis with a dented border (85.3%), mild subungual hyperkeratosis (85.3%), and periungual tissue damage ranging from mild paronychia to excoriations (44.1%) (50). Unlike in nail psoriasis, an erythematous border of onycholysis is infrequently observed (13.8%) in ACD due to artificial nails (25, 50).
For management of ACD due to artificial nails, the causative allergen needs to be identified by patch testing then strictly avoided (51, 52). Topical steroids with a short course of systemic steroids can accelerate recovery (48). In the 2017 multi-centre study, 11.7% of affected nail technicians left their jobs, while 44.2% worked despite the dermatosis. Most consumers’ symptoms resolved on stopping the use of artificial nails, although some consumers reported chronic nail dystrophy and onycholysis.
MIMICS OF INFLAMMATORY NAIL DISORDERS
Onychomycosis
Onychomycosis describes infection of the nail unit by dermatophytes, non-dermatophytes and yeast (53). Similar to trachyonychia, nail psoriasis and nail lichen planus, it presents with yellow, brittle nails (54). It is important to distinguish onychomycosis from such inflammatory nail disorders, as its treatment depends on successful elimination of the causative organism (8).
Key dermoscopic features of onychomycosis include longitudinal striae, colour changes and distal onycholysis with a jagged proximal margin and spikes (Fig. 6) (20, 21, 55–60). Spikes and longitudinal striae are reported as the best diagnostic features by Piraccini et al. (26) and Nada et al. (59), respectively. In previously published comparative analysis of trachyonychia and onychomycosis, distal streaks (odds ratio (OR) 0.005, 95% confidence interval (95% CI) 0.001–0.039), onycholysis (OR 0.056, 95% CI 0.014–0.23) and subungual hyperkeratosis (OR 0.056, 95% CI 0.015–0.21) were significantly associated with onychomycosis over trachyonychia (8). Conversely, red colouration (OR 4.93, 95% CI 1.61–15.1), longitudinal ridging (OR 126, 95% CI 19.5–813), involvement of > 50% of the proximal nail plate width (OR 29.6, 95% CI 6.8–128), involvement of the proximal nail plate (OR 21, 95% CI 4.20–105), splinter haemorrhages (OR 7.67, 95% CI 2.42–24.2), pitting (OR 7.0, 95% CI 1.38–35.5), onychoschizia (OR 4.5, 95% CI 1.09–18.5), red lunula (OR 9.33, 95% CI 1.87–46.7) and thickened ragged cuticles (OR 11.2, 95% CI 3.10–40.7) were more frequently observed in trachyonychia.

Onycholysis
Onycholysis is a disorder characterized by separation of the nail plate from the underlying nail bed (61). It is most commonly associated with physical trauma, but can be related to infection, such as Candida albicans and Pseudomonas, phototoxic drugs including tetracyclines, capecitabine and 5-fluorouracil, contact dermatitis, psoriasis, lichen planus and underlying squamous cell carcinoma (61).
In traumatic onycholysis, the involved nail shows homogenous colouration (yellow or white), but the proximal margin of onycholysis is sharp and linear not jagged (21, 56, 57). Round, dark red haemorrhagic spots and splinter haemorrhages can also be found in traumatic onycholysis (21). Moreover, a regular macular pattern (OR 15.22, p < 0.001), total (OR 14.372, p < 0.001) or partial (OR 16.91, p < 0.001) homogeneous background, and non-classifiable pattern (OR 13.74, p = 0.001) are more commonly found in traumatic onycholysis than onychomycosis (57). Treatment of onycholysis focuses on trauma prevention, such as keeping nails short and dry, and prescribing insoles if patients have uneven flat feet (61).
Nail dystrophy due to systemic amyloidosis
Nail dystrophy in systemic amyloidosis results from amyloid accumulation in the nail plate. It is rare, but may be the first sign of systemic disease (62). As systemic amyloid accumulation can damage multiple organs, including the heart, lungs, kidneys and gastrointestinal tract, prompt recognition of the nail dystrophy is important. However, misdiagnosis can occur, as nails affected by amyloidosis appear similar to trachyonychia and nail lichen planus (63). Therefore, nail matrix biopsy should be initiated in dubious cases.
Amyloid deposits in the nail plate cause onychorrhexis and onychoschizia (63). As amyloid migrates from the nail matrix and nail bed to accumulate in the nail plate, the middle and distal nail plate are mainly affected with relative sparing of the proximal plate (63). Onychorrhexis, onychoschizia and proximal nail sparing coupled with symptoms, such as fatigue, weakness, anorexia, and constipation, should raise suspicion of nail dystrophy due to systemic amyloidosis.
Malignant nail tumours
Nail squamous cell carcinoma (SCC) and amelanotic nail apparatus melanoma (NAM) are important differential diagnoses of inflammatory nail diseases. Nail SCC is a slow-growing malignant tumour, described as “the great mimicker”, as it has a wide range of presentations with non-specific features including localized hyperkeratosis and lateral onycholysis (64, 65). Onychoscopy can aid diagnosis of nail SCC. Its main dermoscopic features include localized hyperkeratosis, polycyclic/fuzzy lesion edges, non-parallel lesion edges, splinter haemorrhages, longitudinal parallel white lines and nail thickening (66, 67).
Amelanotic NAMs are rare and difficult to recognize due to their non-specific clinical appearance (68). They have poor prognoses due to frequent misdiagnosis and association with thick melanomas (68–70). Key dermoscopic features of amelanotic NAM include a polymorphic vascular pattern with milky-red areas, linear irregular vessels, central dotted vessels, hairpin vessels and peripheral blood spots and crusts (Fig. 7) (21, 69, 71–73). Detailed evaluation may reveal micro-Hutchinson’s sign (microscopic brown-black pigmentation of the adjacent cuticle and/or nail fold) in hypomelanotic cases (71–73).

Study limitations
This review has a numbe of limitations. First, the literature on this topic is limited, and further research into identifying features that have diagnostic value is warranted. Secondly, as dermoscopic evaluation of nail disorders is a relatively new field, the terminology used to describe morphological changes is varied across different researchers. Some dermoscopic terms are also used interchangeably with clinical descriptors. This lack of consensus in terminology can create confusion amongst clinicians. Future standardization of terminology is therefore necessary, ideally through a consensus meeting of experts in this field, as conducted for other dermatological diseases (74, 75).
CONCLUSION
Onychoscopy is a useful bedside tool for evaluating inflammatory nail disorders. Recognizing their dermoscopic features has the potential to improve standard of care, optimizing diagnostic accuracy and subsequent management. This review highlights the key dermoscopic features of major inflammatory nail disorders. Further research into the sensitivity and specificity of dermoscopic features and forming a consensus meeting to standardize terminology can consolidate the use of dermoscopy in evaluating inflammatory nail disorders.
The authors have no conflicts of interest to declare.
REFERENCES
- Jacobsen AA, Tosti A. Trachyonychia and twenty-nail dystrophy: a comprehensive review and discussion of diagnostic accuracy. Skin Appendage Disord 2016; 2: 7–13.
- Alkiewicz J. Trachyonychia. Ann Dermatol Venereol 1950; 10: 136–140.
- Starace M, Alessandrini A, Bruni F, Piraccini BM. Trachyonychia: a retrospective study of 122 patients in a period of 30 years. J Eur Acad Dermatol Venereol 2020; 34: 880–884.
- Haber JS, Chairatchaneeboon M, Rubin AI. Trachyonychia: review and update on clinical aspects, histology, and therapy. Skin Appendage Disord 2016; 2: 109–115.
- Tosti A, Fanti PA, Morelli R, Bardazzi F. Trachyonychia associated with alopecia areata: a clinical and pathologic study. J Am Acad Dermatol 1991; 25: 266–270.
- Chernoff KA, Scher RK. Nail disorders: kids are not just little people. Clin Dermatol 2016; 34: 736–741.
- Tosti A, Bardazzi F, Piraccini BM, Fanti PA. Idiopathic trachyonychia (twenty-nail dystrophy): a pathological study of 23 patients. Br J Dermatol 1994; 131: 866–872.
- Jo G, Park JS, Yu DA, Ohn J, Sheu SL, Mun JH. Onychoscopy of trachyonychia: an analysis of 30 patients and comparison with onychomycosis. Br J Dermatol 2018; 179: 491–493.
- Lee YB, Cheon MS, Eun YS, Cho BK, Park YG, Park HJ. Cyclosporin administration improves clinical manifestations and quality of life in patients with 20-nail dystrophy: case series and survey study. J Dermatol 2012; 39: 1064–1065.
- Park JM, Cho HH, Kim WJ, Mun JH, Song M, Kim HS, et al. Efficacy and safety of calcipotriol/betamethasone dipropionate ointment for the treatment of trachyonychia: an open-label study. Ann Dermatol 2015; 27: 371–375.
- Yorulmaz A, Artuz F. A study of dermoscopic features of nail psoriasis. Postepy Dermatol Alergol 2017; 34: 28–35.
- Tan ES, Chong WS, Tey HL. Nail psoriasis: a review. Am J Clin Dermatol 2012; 13: 375–388.
- Augustin M, Reich K, Blome C, Schafer I, Laass A, Radtke MA. Nail psoriasis in Germany: epidemiology and burden of disease. Br J Dermatol 2010; 163: 580–585.
- Jones SM, Armas JB, Cohen MG, Lovell CR, Evison G, McHugh NJ. Psoriatic arthritis: outcome of disease subsets and relationship of joint disease to nail and skin disease. Br J Rheumatol 1994; 33: 834–839.
- Zenke Y, Ohara Y, Kobayashi D, Arai S, Kishimoto M, Okada M, et al. Nail findings in patients with psoriatic arthritis: a cross-sectional study with special reference to transverse grooves. J Am Acad Dermatol 2017; 77: 863–867.
- McGonagle D, Ash Z, Dickie L, McDermott M, Aydin SZ. The early phase of psoriatic arthritis. Ann Rheum Dis 2011; 70: i71–76.
- Raposo I, Torres T. Nail psoriasis as a predictor of the development of psoriatic arthritis. Actas Dermosifiliogr 2015; 106: 452–457.
- Yadav TA, Khopkar US. Dermoscopy to detect signs of subclinical nail involvement in chronic plaque psoriasis: a study of 68 patients. Indian J Dermatol 2015; 60: 272–275.
- Golinska J, Sar-Pomian M, Rudnicka L. Dermoscopic features of psoriasis of the skin, scalp and nails – a systematic review. J Eur Acad Dermatol Venereol 2019; 33: 648–660.
- Bhat YJ, Mir MA, Keen A, Hassan I. Onychoscopy: an observational study in 237 patients from the Kashmir Valley of North India. Dermatol Pract Concept 2018; 8: 283–291.
- Tosti A. Dermoscopy of the hair and nails. 2nd edn. Boca Raton, FL: CRC Press; 2015.
- Nakamura RC, Costa MC. Dermatoscopic findings in the most frequent onychopathies: descriptive analysis of 500 cases. Int J Dermatol 2012; 51: 483–485.
- Iorizzo M, Dahdah M, Vincenzi C, Tosti A. Videodermoscopy of the hyponychium in nail bed psoriasis. J Am Acad Dermatol 2008; 58: 714–715.
- Hashimoto Y, Uyama M, Takada Y, Yoshida K, Ishiko A. Dermoscopic features of nail psoriasis treated with biologics. J Dermatol 2017; 44: 538–541.
- Piraccini BM, Alessandrini A, Starace M. Onychoscopy: dermoscopy of the nails. Dermatol Clin 2018; 36: 431–438.
- Piraccini BM, Bruni F, Starace M. Dermoscopy of non-skin cancer nail disorders. Dermatol Ther 2012; 25: 594–602.
- Rigopoulos D, Baran R, Chiheb S, Daniel CR, 3rd, Di Chiacchio N, Gregoriou S, et al. Recommendations for the definition, evaluation, and treatment of nail psoriasis in adult patients with no or mild skin psoriasis: a dermatologist and nail expert group consensus. J Am Acad Dermatol 2019; 81: 228–240.
- Friedman P, Sabban EC, Marcucci C, Peralta R, Cabo H. Dermoscopic findings in different clinical variants of lichen planus. Is dermoscopy useful? Dermatol Pract Concept 2015; 5: 51–55.
- Nakamura R, Broce AA, Palencia DP, Ortiz NI, Leverone A. Dermatoscopy of nail lichen planus. Int J Dermatol 2013; 52: 684–687.
- Goettmann S, Zaraa I, Moulonguet I. Nail lichen planus: epidemiological, clinical, pathological, therapeutic and prognosis study of 67 cases. J Eur Acad Dermatol Venereol 2012; 26: 1304–1309.
- Grover C, Kharghoria G, Bhattacharya SN. Linear nail bed dyschromia: a distinctive dermoscopic feature of nail lichen planus. Clin Exp Dermatol 2019; 44: 697–699.
- Lipner SR. Nail lichen planus: a true nail emergency. J Am Acad Dermatol 2019; 80: e177–e178.
- Iorizzo M, Tosti A, Starace M, Baran R, Daniel CR, 3rd, Di Chiacchio N, et al. Isolated nail lichen planus: an expert consensus on treatment of the classical form. J Am Acad Dermatol 2020; 83: 1717–1723.
- Maddy AJ, Tosti A. Dermoscopic features of onychotillomania: a study of 36 cases. J Am Acad Dermatol 2018; 79: 702–705.
- Rieder EA, Tosti A. Onychotillomania: an underrecognized disorder. J Am Acad Dermatol 2016; 75: 1245–1250.
- Sidiropoulou P, Sgouros D, Theodoropoulos K, Katoulis A, Rigopoulos D. Onychotillomania: a chameleon-like disorder: case report and review of literature. Skin Appendage Disord 2019; 5: 104–107.
- Schumer MC, Bartley CA, Bloch MH. Systematic review of pharmacological and behavioral treatments for skin picking disorder. J Clin Psychopharmacol 2016; 36: 147–152.
- Patrizi A, Neri I, Fiorentini C, Bonci A, Ricci G. Lichen striatus: clinical and laboratory features of 115 children. Pediatr Dermatol 2004; 21: 197–204.
- Mun JH, Park HJ, Kim HS, Kim SH, Ko HC, Kim BS, et al. Lichen striatus occurring after allogenic peripheral blood stem cell transplantation in an adult with aplastic anemia. Ann Dermatol 2012; 24: 87–89.
- Kim TW, Mun JH, Jwa SW, Song M, Kim HS, Ko HC, et al. Lichen striatus-induced pterygium unguis. Korean J Dermatol 2012; 50: 799–802.
- Tosti A, Peluso AM, Misciali C, Cameli N. Nail lichen striatus: clinical features and long-term follow-up of five patients. J Am Acad Dermatol 1997; 36: 908–913.
- Jakhar D, Kaur I. Onychoscopy of nail involvement in lichen striatus. Indian Dermatol Online J 2018; 9: 360–361.
- Iorizzo M, Rubin AI, Starace M. Nail lichen striatus: is dermoscopy useful for the diagnosis? Pediatr Dermatol 2019; 36: 859–863.
- Coto-Segura P, Costa-Romero M, Gonzalvo P, Mallo-Garcia S, Curto-Iglesias JR, Santos-Juanes J. Lichen striatus in an adult following trauma with central nail plate involvement and its dermoscopy features. Int J Dermatol 2008; 47: 1324–1325.
- Cheon DU, Ro YS, Kim JE. Treatment of nail lichen striatus with intralesional steroid injection: a case report and literature review. Dermatol Ther 2018; 31: e12713.
- Goncalo M, Pinho A, Agner T, Andersen KE, Bruze M, Diepgen T, et al. Allergic contact dermatitis caused by nail acrylates in Europe. An EECDRG study. Contact Derm 2018; 78: 254–260.
- Gregoriou S, Tagka A, Velissariou E, Tsimpidakis A, Hatzidimitriou E, Platsidaki E, et al. The rising incidence of allergic contact dermatitis to acrylates. Dermatitis 2020; 31: 140–143.
- Maddy AJ, Tosti A. What’s New in Nail Disorders. Dermatol Clin 2019; 37: 143–147.
- Mattos Simoes Mendonca M, LaSenna C, Tosti A. Severe onychodystrophy due to allergic contact dermatitis from acrylic nails. Skin Appendage Disord 2015; 1: 91–94.
- Piccolo V, Piraccini BM, Argenziano G, Russo T, Alessandrini A, Starace M. Onychoscopy of allergic contact dermatitis due to artificial nails: a double-centre retrospective study on 35 patients. J Am Acad Dermatol 2020; 83: 1485–1487.
- Rolls S, Chowdhury MM, Cooper S, Cousen P, Flynn AM, Ghaffar SA, et al. Recommendation to include hydroxyethyl (meth)acrylate in the British baseline patch test series. Br J Dermatol 2019; 181: 811–817.
- Raposo I, Lobo I, Amaro C, Lobo ML, Melo H, Parente J, et al. Allergic contact dermatitis caused by (meth)acrylates in nail cosmetic products in users and nail technicians – a 5-year study. Contact Derm 2017; 77: 356–359.
- Lipner SR, Scher RK. Onychomycosis: clinical overview and diagnosis. J Am Acad Dermatol 2019; 80: 835–851.
- Lipner SR, Scher RK. Onychomycosis: treatment and prevention of recurrence. J Am Acad Dermatol 2019; 80: 853–867.
- Jesus-Silva MA, Fernandez-Martinez R, Roldan-Marin R, Arenas R. Dermoscopic patterns in patients with a clinical diagnosis of onychomycosis-results of a prospective study including data of potassium hydroxide (KOH) and culture examination. Dermatol Pract Concept 2015; 5: 39–44.
- Piraccini BM, Balestri R, Starace M, Rech G. Nail digital dermoscopy (onychoscopy) in the diagnosis of onychomycosis. J Eur Acad Dermatol Venereol 2011; 27: 509–513.
- Ramos Pinheiro R, Dias Domingues T, Sousa V, Galhardas C, Apetato M, Lencastre A. A comparative study of onychomycosis and traumatic toenail onychodystrophy dermoscopic patterns. J Eur Acad Dermatol Venereol 2019; 33: 786–792.
- Maatouk I, Haber R, Benmehidi N. Onychoscopic evaluation of distal and lateral subungual onychomycosis: a cross-sectional study in Lebanon. Curr Med Mycol 2019; 5: 41–44.
- Nada EEA, El Taieb MA, El-Feky MA, Ibrahim HM, Hegazy EM, Mohamed AE, et al. Diagnosis of onychomycosis clinically by nail dermoscopy versus microbiological diagnosis. Arch Dermatol Res 2020; 312: 207–212.
- Abdallah NA, Said M, Mahmoud MT, Omar MA. Onychomycosis: Correlation between the dermoscopic patterns and fungal culture. J Cosmet Dermatol 2020; 19: 1196–1204.
- Zaias N, Escovar SX, Zaiac MN. Finger and toenail onycholysis. J Eur Acad Dermatol Venereol 2015; 29: 848–853.
- Prat C, Moreno A, Vinas M, Jucgla A. Nail dystrophy in primary systemic amyloidosis. J Eur Acad Dermatol Venereol 2008; 22: 107–109.
- Jo G, Shin DY, Mun JH. Systemic amyloidosis-induced nail dystrophy. J Dtsch Dermatol Ges 2019; 17: 1057–1059.
- Lecerf P, Richert B, Theunis A, André J. A retrospective study of squamous cell carcinoma of the nail unit diagnosed in a Belgian general hospital over a 15-year period. J Am Acad Dermatol 2013; 69: 253–261.
- Starace M, Alessandrini A, Dika E, Piraccini BM. Squamous cell carcinoma of the nail unit. Dermatol Pract Concept 2018; 8: 238–244.
- Teysseire S, Dalle S, Duru G, Phan A, Debarbieux S, Poulhalon N, et al. Dermoscopic features of subungual squamous cell carcinoma: a study of 44 cases. Dermatology (Basel) 2017; 233: 184–191.
- Dika E, Fanti PA, Patrizi A, Misciali C, Vaccari S, Piraccini BM. Mohs surgery for squamous cell carcinoma of the nail unit: 10 years of experience. Dermatol Surg 2015; 41: 1015–1019.
- Thai KE, Young R, Sinclair RD. Nail apparatus melanoma. Australas J Dermatol 2001; 42: 71–81; quiz 82–73.
- Phan A, Dalle S, Touzet S, Ronger-Savlé S, Balme B, Thomas L. Dermoscopic features of acral lentiginous melanoma in a large series of 110 cases in a white population. Br J Dermatol 2010; 162: 765–771.
- Phan A, Touzet S, Dalle S, Ronger-Savlé S, Balme B, Thomas L. Acral lentiginous melanoma: a clinicoprognostic study of 126 cases. Br J Dermatol 2006; 155: 561–569.
- Starace M, Dika E, Fanti PA, Patrizi A, Misciali C, Alessandrini A, et al. Nail apparatus melanoma: dermoscopic and histopathologic correlations on a series of 23 patients from a single centre. J Eur Acad Dermatol Venereol 2018; 32: 164–173.
- Menzies SW, Kreusch J, Byth K, Pizzichetta MA, Marghoob A, Braun R, et al. Dermoscopic evaluation of amelanotic and hypomelanotic melanoma. Arch Dermatol 2008; 144: 1120–1127.
- Argenziano G, Zalaudek I, Corona R, Sera F, Cicale L, Petrillo G, et al. Vascular structures in skin tumors: a dermoscopy study. Arch Dermatol 2004; 140: 1485–1489.
- Errichetti E, Zalaudek I, Kittler H, Apalla Z, Argenziano G, Bakos R, et al. Standardization of dermoscopic terminology and basic dermoscopic parameters to evaluate in general dermatology (non-neoplastic dermatoses): an expert consensus on behalf of the International Dermoscopy Society. Br J Dermatol 2020; 182: 454–467.
- Kittler H, Marghoob AA, Argenziano G, Carrera C, Curiel-Lewandrowski C, Hofmann-Wellenhof R, et al. Standardization of terminology in dermoscopy/dermatoscopy: results of the third consensus conference of the International Society of Dermoscopy. J Am Acad Dermatol 2016; 74: 1093–1106.