ORIGINAL REPORT
Role of Mannose-binding Lectin and Association with Microbial Sensitization in a Cohort of Patients with Atopic Dermatitis
Emma BELFRAGE1, Camilla L. JINNESTÅL1, Andreas JÖNSEN2, Anders BENGTSSON2, Anna ÅKESSON3, Artur SCHMIDTCHEN1,4 and Andreas SONESSON1
1Division of Dermatology and Venereology, 2Section of Rheumatology, Department of Clinical Sciences Lund, Lund University, Skåne University Hospital, 3Clinical Studies Sweden, Forum South, Skåne University Hospital, Lund, Sweden and 4Copenhagen Wound Healing Center, Bispebjerg Hospital, Department of Biomedical Sciences, University of Copenhagen, Copenhagen, Denmark
Abstract
Atopic dermatitis is a relapsing inflammatory skin condition, in which bacteria, fungi and viruses may colonize the skin and aggravate the condition. Mannose-binding lectin is part of the innate immune system. Polymorphism in the mannose-binding lectin gene can result in deficiency of mannose-binding lectin, which may affect defence against microbes. The aim of this study was to investigate whether polymorphisms in the mannose-binding lectin gene affect the extent of sensitization to common skin microbes, the skin barrier function, or the severity of the disease in a cohort of patients with atopic dermatitis. Genetic testing of mannose-binding lectin polymorphism was performed in 60 patients with atopic dermatitis. The disease severity, skin barrier function, and serum levels of specific immunoglobulin E against skin microbes were measured. In patients with low mannose-binding lectin genotype (group 1) 6 of 8 (75%) were sensitized to Candida albicans, compared to 14 of 22 (63.6%) patients with intermediate mannose-binding genotype (group 2) and 10 of 30 (33.3%) patients with high mannose-binding genotype (group 3). Group 1 (low mannose-binding lectin) was more likely to be sensitized to Candida albicans compared with group 3 (high mannose-binding lectin) (odds ratio 6.34, p-value 0.045). In this cohort of patients with atopic dermatitis, mannose-binding lectin deficiency was associated with increased sensitization to Candida albicans.
Key words: atopic dermatitis; mannose binding lectin; sensitization immunological; Candida albicans.
SIGNIFICANCE
Atopic dermatitis is a chronic inflammatory skin disease. Mannose-binding lectin is part of the innate immune system, participating in defence against skin microbes. The aim of this study was to investigate how genes coding for mannose-binding lectin affect the course of atopic dermatitis and the sensitization to skin microbes commonly present on the skin. This study shows that mannose-binding lectin gene polymorphism was associated with increased sensitization to Candida in a cohort of patients with atopic dermatitis. Sensitization to skin microbes in patients with atopic dermatitis may aggravate the disease.
Citation: Acta Derm Venereol 2023; 103: adv2405. DOI https://doi.org/10.2340/actadv.v103.2405.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Dec 15, 2022; Published: Mar 30, 2023
Corr: Emma Belfrage, Division of Dermatology and Venereology, Section of Rheumatology, Department of Clinical Sciences Lund, Lund University, Skåne University Hospital, Lasarettsgatan 15, SE-221 85 Lund, Sweden. E-mail: emma.belfrage@med.lu.se
Competing interests and funding: EB received lecturing fees from Novartis. AS received lecturing and consulting fees from Sanofi, Pfizer, Abbvie and Leo Pharma. These payments were made to these authors’ affiliated institutions.
The authors have no conflicts of interest to declare.
INTRODUCTION
Atopic dermatitis (AD) is a chronic inflammatory skin condition characterized by dry skin and itching. The pathogenesis of AD involves defects in the skin barrier, and impaired innate and adaptive immunity. Environmental factors can also affect the severity of AD (1). Patients with AD have an increased susceptibility to cutaneous colonization and infection with bacteria, fungi and viruses. Approximately 90% of patients with AD are colonized with Staphylococcus aureus (S. aureus), which, once present on the skin, can mediate multiple inflammatory cascades and induce immunoglobulin E (IgE)-specific responses (2). These IgE levels have been shown to correspond with disease severity (3, 4). Candida albicans (C. albicans) is a member of the normal microbiota of the mucous membranes and may also colonize the skin. Candida species have been isolated more commonly from the skin of patients with AD compared with those with psoriasis and healthy control subjects (5). Excessive growth of yeasts or inappropriate host immunoreactivity may cause skin disorders, such as pityriasis versicolor, folliculitis and seborrhoeic disease. The yeasts Malassezia and C. albicans may aggravate the disease due to an allergic reaction, particularly in the head and neck type of AD (6, 7). Specific IgE to C. albicans is shown to be more frequent in patients with AD compared to healthy controls (8). Treatment with systemic ketoconazole in patients with AD has shown a significant decrease in specific IgE to Malassezia and C. albicans, as well as a decrease in disease activity (9, 10).
Mannose-binding lectin (MBL) is an acute phase reactant produced by the liver, and is an important component of the innate immunity. MBL recognizes specific sugar combinations that occur on the surface of many pathogens, which leads to opsonization, phagocytosis and complement activation (11–13). MBL is encoded by a gene located on chromosome 10, in which several polymorphisms are present. Polymorphism of the structural MBL gene and the associated promotor region result in failure to assemble functional protein and serum MBL-deficiency (14, 15). Low levels of MBL in serum due to genetic variations are found to be 10-15% (13–15). The condition has been associated with autoimmune diseases and increased susceptibility to infections under certain circumstances (15). MBL has been shown to play a part in our defence against Candida species (16, 17).
The role of MBL in dermatological disease is sparsely investigated, and there are conflicting reports on the association between polymorphism of the MBL gene and AD.Brandão et al. (18) demonstrated that the MBL allelic variants responsible for defective MBL protein levels were significantly more common in children with AD than in healthy adults. Carrera et al found that children with AD had a significantly higher frequency of variant alleles coding for deficient production of MBL compared with a healthy control group, but the variant alleles were not associated with severity of AD (19). Hashimoto et al. did not find any associations between MBL polymorphisms and AD (20).
This study aimed to investigate the role of MBL polymorphism in AD in relation to sensitization to skin microbes. A further aim was analyse whether MBL polymorphisms in patients with AD correlated with disease severity or a disrupted skin barrier function. A disrupted skin barrier function in AD skin results in increased transepidermal water loss (TEWL) and correlates with increased severity of AD (21, 22).
MATERIALS AND METHODS
Subjects
A total of 65 adult patients with AD were recruited when visiting the Dermatology Clinic in Lund, Skåne University Hospital, Sweden, between 2011 and 2014. Diagnosis was verified using the diagnostic criteria for AD (23, 24). It was possible to perform genetic testing for MBL polymorphism in 60 patients, and the remaining 5 were excluded from further analyses in the study (see Appendix S1).
Immunological analyses and serum analyses of mannose-binding lectin
Sensitization to skin-associated microorganisms was measured. Specific IgE ≥ 0.35k U/L was considered positive. MBL serum concentrations were analysed. MBL < 100 μg/l was considered MBL deficiency (see Appendix S1).
Mannose-binding lectin gene polymorphism
Variants of MBL due to mutations at codon 52 (D), 54 (B), and 57 (C) in exon 1 of the MBL structural gene and promoter variants at position –550 (H/L) and –221 (X/Y) were determined by allele-specific PCR amplification. The wild-type structural allele is designated A, while 0 is a description of the mutant alleles B, C and D. Based on previously described associations between MBL genotype and MBL serum concentrations, which were confirmed in 200 healthy controls, the MBL genotypes were divided into 3 groups (14, 15, 25, 26). Group 1 (low MBL) comprised patients with 2 structural mutant alleles (0/0) or on 1 haplotype a structural mutant allele together with another haplotype containing an LX promoter and the wild-type structural allele (ALX/0) and were expected to have serum MBL deficiency. Group 2 (intermediate MBL) comprised patients with the promoter LX conferring low serum MBL on both haplotypes but with normal structural alleles (ALX/ALX), or, alternatively, haplotypes with 1 mutant and 1 wild-type structural allele with a non-LX promoter together with the wild-type allele. Group 3 (high MBL) included patients with the A/A genotype and at least 1 non-LX promoter. Groups 2 and 3 were expected to have no serum MBL deficiency (15, 25, 26) (see Appendix S1).
Transepidermal water loss
TEWL was measured as described previously (27) (see Appendix S1).
Assessment of disease severity
Disease severity of AD was determined (28, 29) (see Appendix S1).
Statistical analysis
See Appendix S1.
RESULTS
Characteristics of the patients with atopic dermatitis, mannose-binding lectin polymorphism and correlating mannose-binding lectin groups
Genetic testing for MBL gene polymorphism was performed in 60 patients. The patients were divided into 3 groups according to their MBL polymorphism status (Table SI). Eight patients fulfilled the criteria of group 1 (low MBL), considered to have a MBL deficiency. Twenty-two patients were placed in group 2 (intermediate MBL), while 30 patients met the criteria for group 3 (high MBL). The frequency of a MBL genotype associated with serum MBL deficiency (low MBL) was 13% (8/60). There were more women in comparison with men in all groups; however, the proportion of women in the MBL groups was equally distributed. There was a wide range of total IgE in all groups. The MBL groups were similar in TEWL. Group 1 (low MBL) had a SCORing Atopic Dermatitis (SCORAD) median of 26, and group 2 (intermediate MBL) and group 3 (high MBL) had a SCORAD median of 33.5 and 27, respectively. The prevalence of head and neck dermatitis was 87.5% in group 1 (low MBL), 77% in group 2 (intermediate MBL) and 60% group 3 (high MBL) (Tables SII and SIV).
Mannose-binding lectin genotype groups and correlating MBL serum concentrations
As expected, the patients in group 1 (low MBL) showed MBL deficiency, even though 1 patient with the polymorphism ALX DHY had a MBL serum concentration > 100 μg/L. In group 2 (intermediate MBL) and group 3 (high MBL) the median values of MBL serum concentrations showed no MBL deficiency, although 3 patients with the polymorphism AHY BLY and 1 patient with genotype ALY BLY had serum concentrations of MBL < 100 μg/l (Fig. 1).
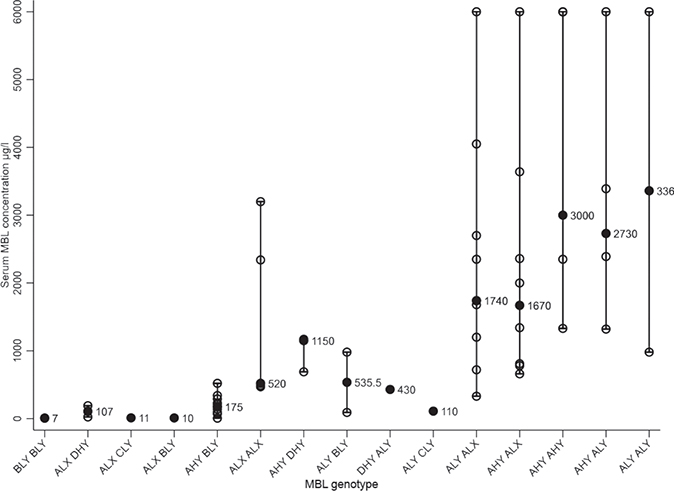
Fig. 1. Mannose-binding lectin (MBL) serum concentrations according to MBL genotype. Black dots indicate the median MBL concentrations for each MBL genotype, which are also printed out in the figure. Each individual MBL serum concentration is represented as white dot and the range is shown as a longitudinal line. The MBL genotypes are explained in Table SI.
Mannose-binding lectin genotype groups and sensitiza-tion to Candida albicans
The results showed a patient frequency with IgE sensitivity to C. albicans of 75% (6/8) in group 1 (low MBL), 63.6% (14/22) in the group 2 (intermediate MBL) and 33.3% (10/30) in the group 3 (high MBL) (Table SII). There was a significant difference between the different MBL groups concerning sensitization to C. albicans (p - value 0.031, Table I). However, the result of serum-specific IgE sensitization to the other skin-associated microorganisms showed no differences between the MBL groups (Table SIII). To further investigate the assoction between group 1 (low MBL) and sensitization to C. albicans, logistic regression models were constructed (Table II). The results revealed that AD patients in group 1 (low MBL) were 6 times more likely to be sensitized to C. albicans compared with group 3 (high MBL) (odds ratio (OR) = 6.00 unadjusted for age and sex, p-value 0.047) (Table II). This was also the case when adjusted for age and sex (OR = 6.34, p-value 0.045). Moreover, it was 22 times more likely that the group with a severe disease (SCORAD > 50) was sensitized to C. albicans compared with the group with a less severe disease (SCORAD < 25) (Table II).
| Model | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||||||||||||||||||
| Variable | OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | ||||||||||
| MBL | ||||||||||||||||||||||||||
| Group 1a | 6.00 (1.02–35.27) | 0.047 | 17.49 (1.95–156.52) | 0.010 | 6.33 (1.07–37.42) | 0.042 | 5.50 (0.91–33.09) | 0.063 | 6.34 (1.04–38.62) | 0.045 | 17.92 (1.98–162.01) | 0.010 | 6.62 (1.08–40.63) | 0.041 | 6.10 (0.97–38.29) | 0.054 | ||||||||||
| Group 2b | 3.50 (1.10–11.09) | 0.033 | 4.40 (1.07–18.71) | 0.040 | 3.01 (0.91–9.92) | 0.070 | 3.32 (1.03–10.67) | 0.044 | 3.25 (1.00–10.54) | 0.050 | 4.31 (1.02–18.27) | 0.047 | 2.73 (0.81–9.22) | 0.107 | 3.19 (0.98–10.46) | 0.055 | ||||||||||
| Group 3c | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||||||||||||||||||
| SCORAD | ||||||||||||||||||||||||||
| <25 | Ref. | Ref. | ||||||||||||||||||||||||
| 25–50 | 9.60 (1.98–46.50) | 0.005 | 9.75 (1.90–50.19) | 0.006 | ||||||||||||||||||||||
| < 50 | 22.47 (3.64–38.51) | 0.001 | 21.48 (3.36–137.19) | 0.001 | ||||||||||||||||||||||
| TEWL, g/m2/h | 1.03 (0.97–1.08) | 0.377 | 1.03 (0.97–1.09) | 0.332 | ||||||||||||||||||||||
| Head and neck | 1.42 (0.43–4.72) | 0.568 | 1.15 (0.33–3.23) | 0.967 | ||||||||||||||||||||||
| aLow MBL. bIntermediate MBL cHigh MBL Logistic regression models were used to calculate odds ratio (OR), models 5–8 were adjusted for age and sex, models 4 and 8 were adjusted for head and neck dermatitis. Ref.: reference. 95% CI: 95% confidence interval. |
||||||||||||||||||||||||||
DISCUSSION
This study characterized a cohort of patient with AD concerning their MBL genotypes and the associations with sensitization to skin microbes that often colonize the skin. The results indicate that MBL genotypes considered to result in MBL deficiency were associated with sensitization to C. albicans, but not to any other of the investigated skin microbes (S. aureus, Malassezia). One hypothesis for this might be the role MBL play in the innate immunity and in the defence against C. albicans (11, 13, 16, 17). Speculatively, patients with AD and MBL deficiency may be more prone to be colonized with C. albicans and, hence, as a result, might have a higher frequency of sensitization to C. albicans. However, the frequency of skin colonization with C. albicans was not studied in this cohort. AD is a genetically complex disease in which impaired innate immunity plays a significant part and these results could be of interest for the group of patients with AD with head and neck dermatitis in whom colonization with fungi is more frequent.
Patients with severe AD can have high levels of total IgE and sensitization to numerous allergens, detected by the ImmunoCAP™ system (Phadia AB, Uppsala, Sweden), in which a positive specific IgE may be less clinically relevant. The threshold for positive allergen-specific IgE in this study was set at > 0.35kU/l which could be considered a low cut-off value. However, based at international consensus for allergen-specific IgE test, a value of > 0.35kU/l as cut-off was used. Moreover, of the 30 patients who were sensitized to C. albicans, only 3 patients had CAP class I and the rest had CAP class II or higher, suggesting that the senitization had an clinical impact (Table SIV). The association in this cohort between group 1 (low MBL) and sensitization to C. albicans might be an epiphenomenon. However, in this cohort, group 1 (low MBL) was associated only with IgE sensitization to C albicans, and not to any other skin microbes, suggesting that the MBL gene polymorphism might be of importance.
Carrera et al. (19) concluded that low or deficient MBL serum levels determined genetically may contribute to the predisposition for AD, but not for disease severity. When the role of MBL genotype on disease severity, measured as SCORAD, and barrier function, measured as TEWL, were investigated in this cohort, no significant influence of MBL polymorphism on TEWL and SCORAD was observed. However, the number of patients in this cohort was limited and further studies are warranted to study the role of MBL gene polymorphism in severity of AD.
The interplay between the polymorphism of the MBL gene in the structural and promotor regions and the effect on MBL serum concentrations has been studied in different ethnicities (14, 15). In this Swedish cohort of patients with AD, the classification of MBL genotypes was based on a Swedish control group of 200 individuals (15). The MBL genotypes were categorized into 3 groups as previously performed by Jönsen et al. (25, 26), in which group 1 (low MBL) was expected to be MBL deficient and group 2 (intermediate MBL) and group 3 (high MBL) were considered to be MBL sufficient. In this cohort of patients with AD, the prevalence of MBL genotypes with considered association with MBL deficiency was 13% (8/60). Within the same genotype there was a range of serum MBL concentration, as described previously (14, 15). This makes the classification of the MBL genotypes challenging and highlights the need for further studies of the MBL gene.
The pathogenesis of AD involves several factors, which include innate immunity as an important part. Therefore, further studies are warranted to study the role of MBL in the context of AD, and association with the skin microbiota.
ACKNOWLEDGEMENTS
We thank Birgitta Gullstrand, Section of Rheumatology, Department of Clinical Sciences Lund, Lund University, Skåne University Hospital, Lund, Sweden for technical support. We are grateful for the support from Professor Ove Bäck and Christer Hansson, Department of Dermatology and Venereology, Lund University, Skåne University Hospital, Lund, Sweden.
This work was supported by a grant from the Welander and Finsen Research Foundation (Hudfonden), the Swedish Research Council (project 2017-02341, 2020-02016) and the Swedish Government Funds for Clinical Research (ALF).
REFERENCES
- Bieber T. Atopic dermatitis. Ann Dermatol 2010; 22: 125–137.
- Leung DY. Infection in atopic dermatitis. Curr Opin Pediatr 2003; 15: 399–404.
- Sohn MH, Kim CH, Kim WK, Jang GC, Kim KE. Effect of staphylococcal enterotoxin B on specific antibody production in children with atopic dermatitis. Allergy Asthma Proc 2003; 24: 67–71.
- Bunikowski R, Mielke M, Skarabis H, Herz U, Bergmann RL, Wahn U, et al. Prevalence and role of serum IgE antibodies to the Staphylococcus aureus-derived superantigens SEA and SEB in children with atopic dermatitis. J Allergy Clin Immunol 1999; 103: 119–124.
- Arzumanyan VG, Magarshak OO, Semenov BF. Yeast fungi in patients with allergic diseases: species variety and sensitivity to antifungal drugs. Bull Exp Biol Med 2000; 129: 601–604.
- Faergemann J. Atopic dermatitis and fungi. Clin Microbiol Rev 2002; 15: 545–563.
- Sonesson A, Bartosik J, Christiansen J, Roscher I, Nilsson F, Schmidtchen A, et al. Sensitization to skin-associated microorganisms in adult patients with atopic dermatitis is of importance for disease severity. Acta Derm Venereol 2013; 93: 340–345.
- Back O, Scheynius A, Johansson SG. Ketoconazole in atopic dermatitis: therapeutic response is correlated with decrease in serum IgE. Arch Dermatol Res 1995; 287: 448–451.
- Back O, Bartosik J. Systemic ketoconazole for yeast allergic patients with atopic dermatitis. J Eur Acad Dermatol Venereol 2001; 15: 34–38.
- Darabi K, Hostetler SG, Bechtel MA, Zirwas M. The role of Malassezia in atopic dermatitis affecting the head and neck of adults. J Am Acad Dermatol 2009; 60: 125–136.
- van Asbeck EC, Hoepelman AI, Scharringa J, Herpers BL, Verhoef J. Mannose binding lectin plays a crucial role in innate immunity against yeast by enhanced complement activation and enhanced uptake of polymorphonuclear cells. BMC Microbiol 2008; 8: 229.
- Wang M, Chen Y, Zhang Y, Zhang L, Lu X, Chen Z. Mannan-binding lectin directly interacts with Toll-like receptor 4 and suppresses lipopolysaccharide-induced inflammatory cytokine secretion from THP-1 cells. Cell Mol Immunol 2011; 8: 265–275.
- Heitzeneder S, Seidel M, Forster-Waldl E, Heitger A. Mannan-binding lectin deficiency – Good news, bad news, doesn’t matter? Clin Immunol 2012; 143: 22–38.
- Madsen HO, Garred P, Thiel S, Kurtzhals JA, Lamm LU, Ryder LP, et al. Interplay between promoter and structural gene variants control basal serum level of mannan-binding protein. J Immunol 1995; 155: 3013–3020.
- Carlsson M, Sjoholm AG, Eriksson L, Thiel S, Jensenius JC, Segelmark M, et al. Deficiency of the mannan-binding lectin pathway of complement and poor outcome in cystic fibrosis: bacterial colonization may be decisive for a relationship. Clin Exp Immunol 2005; 139: 306–313.
- Hammad NM, El Badawy NE, Ghramh HA, Al Kady LM. Mannose-binding lectin: a potential therapeutic candidate against candida infection. Biomed Res Int 2018; 2018: 2813737.
- Lillegard JB, Sim RB, Thorkildson P, Gates MA, Kozel TR. Recognition of Candida albicans by mannan-binding lectin in vitro and in vivo. J Infect Dis 2006; 193: 1589–1597.
- Brandao LA, Guimaraes RL, Carrera M, Milanese M, Segat L, Luiz de Lima-Filho J, et al. MBL2 functional allelic variants and increased risk for the development of atopic dermatitis in Brazilian children. Arch Dermatol 2008; 144: 412–413.
- Carrera MC, Moura P, Crovella S, de Souza PR, de Alencar LC, Sarinho E. High polymorphism of the MBL2 gene in patients with atopic dermatitis. Ann Allergy Asthma Immunol 2010; 105: 39–42.
- Hashimoto S, Nakamura K, Oyama N, Kaneko F, Fujita T, Tsunemi Y, et al. Mannose-binding lectin (MBL) single nucleotide polymorphism is not associated with atopic dermatitis in Japanese patients. J Dermatol 2005; 32: 1038–1040.
- Kim DW, Park JY, Na GY, Lee SJ, Lee WJ. Correlation of clinical features and skin barrier function in adolescent and adult patients with atopic dermatitis. Int J Dermatol 2006; 45: 698–701.
- Gupta J, Grube E, Ericksen MB, Stevenson MD, Lucky AW, Sheth AP, et al. Intrinsically defective skin barrier function in children with atopic dermatitis correlates with disease severity. J Allergy Clin Immunol 2008; 121: 725–730.e2.
- Brenninkmeijer EE, Schram ME, Leeflang MM, Bos JD, Spuls PI. Diagnostic criteria for atopic dermatitis: a systematic review. Br J Dermatol 2008; 158: 754–765.
- Williams HC, Burney PG, Pembroke AC, Hay RJ. The U.K. Working Party’s Diagnostic Criteria for Atopic Dermatitis. III. Independent hospital validation. Br J Dermatol 1994; 131: 406–416.
- Jönsen A, Bengtsson AA, Sturfelt G, Truedsson L. Analysis of HLA DR, HLA DQ, C4A, FcgammaRIIa, FcgammaRIIIa, MBL, and IL-1Ra allelic variants in Caucasian systemic lupus erythematosus patients suggests an effect of the combined FcgammaRIIa R/R and IL-1Ra 2/2 genotypes on disease susceptibility. Arthritis Res Ther 2004; 6: R557–R562.
- Jönsen A, Gullstrand B, Guner N, Bengtsson AA, Nived O, Truedsson L, et al. Genetically determined mannan-binding lectin deficiency is of minor importance in determining susceptibility to severe infections and vascular organ damage in systemic lupus erythematosus. Lupus 2007; 16: 245–253.
- Pinnagoda J, Tupker RA, Agner T, Serup J. Guidelines for transepidermal water loss (TEWL) measurement. A report from the Standardization Group of the European Society of Contact Dermatitis. Contact Dermatitis 1990; 22: 164–178.
- Schmitt J, Langan S, Williams HC, European Dermato-Epidemiology N. What are the best outcome measurements for atopic eczema? A systematic review. J Allergy Clin Immunol 2007; 120: 1389–1398.
- Severity scoring of atopic dermatitis: the SCORAD index. Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology 1993; 186: 23–31.