Eccrine porocarcinoma (EPC) is a rare malignant neoplasm that arises from the intraepidermal part of the sweat duct gland and frequently manifests as a solitary nodule or plaque on the limbs or the head and neck area in elderly patients (1, 2). EPCs exhibit invasive growth and may have high morbidity and mortality potential (1, 2). Because of its low incidence, lack of distinct clinical features, variable histomorphological appearance, and absence of specific immunohistochemical methods, EPC diagnosis is often challenging (1–4). Recently, highly recurrent YAP1 and NUTM1 gene rearrangements have been described in cases of poromas and EPCs, highlighting the potential usefulness of immunohistochemical and molecular studies in the diagnosis of these neoplasms (3).
CASE REPORT
Clinical history. An otherwise healthy 51-year-old woman was referred to the department of Dermatology for evaluation of a 3-month history of enlarged and swollen lymph nodes on her right groin. Past medical history included the diagnosis of an EPC on the right popliteal fossa that was completely excised 15 years previously (at this time, a sentinel lymph node biopsy was not considered), with no signs of locoregional recurrence after a 10-year clinical follow-up period (Fig. 1a–b). Physical examination disclosed several tender erythematous subcutaneous nodules located on the right groin corresponding to enlarged lymph nodes. The rest of the physical examination, including a complete gynaecological and pelvic examination, was unremarkable, and no signs of local recurrence were detected in the postsurgical scar on the right popliteal area.
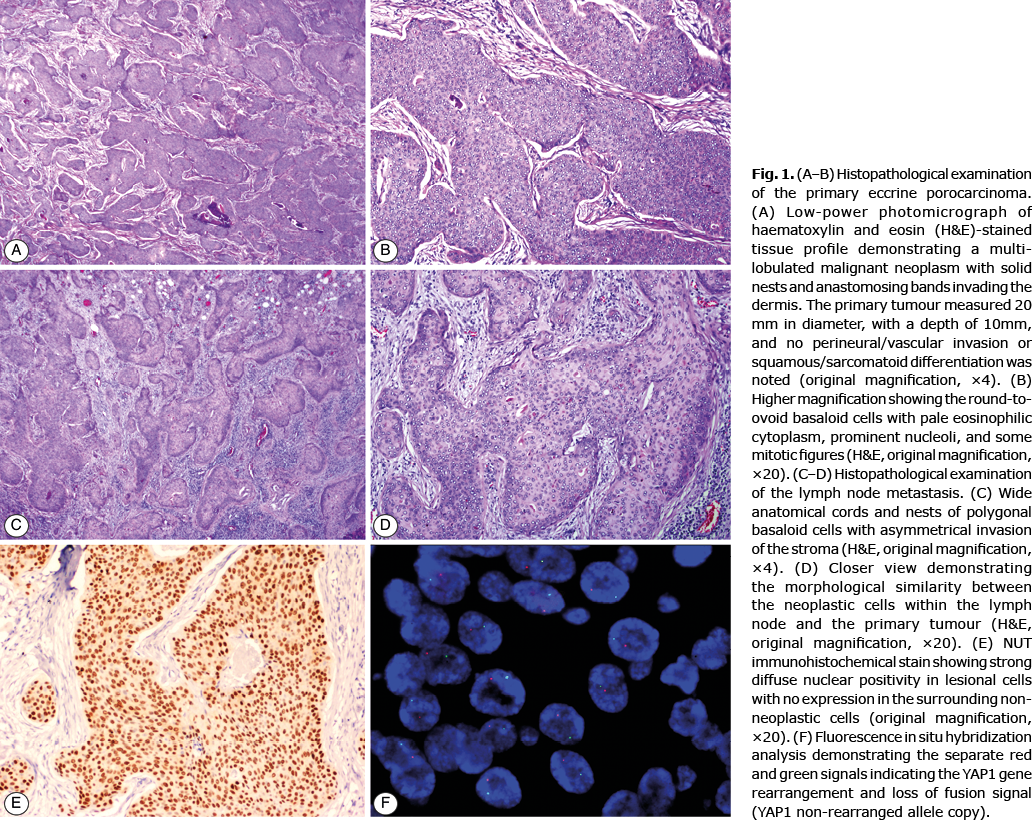
Histological, immunohistochemical and fluorescence in situ hybridization findings. A core-needle biopsy was obtained and the observed histopathological features were diagnosed as consistent with diffuse infiltration by moderately-differentiated squamous cell carcinoma (SCC). A positron emission tomography-computed tomography ruled out visceral involvement and no other hypermetabolic foci were detected. An inguinal lymphadenectomy was performed, and histopathological examination showed a diffuse lymph node infiltration by large pleomorphic round and oval cells arranged in groups and lobules with capsular rupture in 2 out of 6 lymph nodes removed (Fig. 1c, d). These malignant cells expressed the immunohistochemical markers cytokeratin AE1/AE3 and epithelial membrane antigen (EMA). YAP (C-terminus) and NUT immunohistochemistry were also performed on both the original EPC cutaneous specimen and the lymph node sample. The same results were observed in both specimens: a diffuse strong nuclear positivity for NUT, along with a total loss of YAP1 expression, which was consistent with the presence of an underlying YAP1-NUTM1 translocation (Fig. 1e). The fluorescence in situ hybridization (FISH) analysis confirmed the YAP1 gene rearrangement (Fig. 1f).
Treatment and follow-up. With the diagnosis of surgically resected metastatic EPC, and based on a multidisciplinary tumour board decision, adjuvant radiotherapy on inguinal region was recommended and the patient is currently undergoing a close clinical and imaging surveillance every 3 months.
DISCUSSION
New molecular pathways involved in the pathogenesis of poroid neoplasms have been described recently (3, 5–7). Thus, cytogenetic translocations involving YAP1, specifically the YAP1-MAML2 and YAP1-NUTM1 fusions, have been identified in approximately 89% of poromas and 64% of EPCs (3). The tumorigenic role of YAP1 fusions might be explained by the activation of transcription factors and promotion of anchorage-independent growth in epithelial cells (3). Such genomic rearrangements seem to be specific of poromas and EPCs, since YAP1 fusions have not been identified in other skin neoplasms (3). The current patient, to our knowledge, represents the first description of YAP1-NUTM1 fusion in a metastatic EPC, demonstrating the presence of the genetic rearrangement in both the primary tumour and its metastasis. As in the current patient, the detection of a YAP1-NUTM1 gene fusion in cases of metastatic lymph node involvement from a malignant neoplasm with unknown origin favours the poroid nature of the primary tumour.
The demonstration of specific molecular rearrangements in poroid neoplasms also represents a diagnostic opportunity to use immunochemistry as a useful diagnostic tool for these neoplasms. Thereby, recent studies have postulated that NUT immunohistochemistry might be considered as a potential histological marker of poromas and EPCs, since NUT would be overexpressed in YAP1-NUTM1-rearranged tumours (3, 5, 8). In this sense, it has been shown that this marker could have a high specificity (close to 100%) in the diagnosis of poroid neoplasms, since other skin tumours (including histological mimics of EPC, such as SCC or hidradenocarcinoma) do not express NUT (5, 8). Therefore, and given the high degree of concordance between the molecular and immunohistochemical results found in recent investigations (3, 5), NUT immunohistochemistry may represent a simpler, faster and more accessible technique than the molecular approach to better characterize EPC cases.
EPC represents a cutaneous neoplasm with high rates of extracutaneous spread. It has been demonstrated that 22.3% of EPC cases present as metastatic disease at the time of diagnosis, including regional lymph node (17%), distant (3.9%) and cutaneous metastases (1.5%) (1). Overall survival of patients with metastatic EPC has not been well characterized, but is probably poor (9). Moreover, the time from EPC diagnosis until the development of metastases is variable, but in most cases it has been estimated to be less than 1 year (1). Nevertheless, the present case demonstrates that late metastases could develop in EPC cases. Therefore, a long-term follow-up, with regular skin and lymph node examination, seems advisable for this malignancy.
Given its low incidence, little guidance is available in the literature regarding EPC management. For local disease, surgical resection represents the main treatment and may include wide local excision or Mohs micrographic surgery (10). Sentinel lymph node biopsy should also be considered in EPC cases exhibiting high-risk features (1). Systemic treatment of unresectable metastatic EPC has not been established, and several therapeutic regimens have been postulated in different case reports with variable results (radiotherapy, chemotherapy-based regimens, cetuximab, anti-PD1 agents, among others) (9, 11). The identification of the presence of YAP1 fusions in EPC cases might also have therapeutic implications, representing a potential therapeutic target for this rare malignancy.
In conclusion, we present here a unique case of an aggressive EPC that developed regional lymph node metastasis after 15 years from the treatment of the primary tumour, in which the immunohistochemical pattern and FISH results were consistent with a YAP1-NUTM1 rearrangement. A better understanding of the molecular pathways involved in the development of these neoplasms would help not only to improve the characterization and diagnosis of EPC cases, but also to the development of targeted therapies in the era of personalized medicine.
ACKNOWLEDGEMENTS
The authors would like to thank Evelyn Andrades and Maria Rodriguez-Rivera for their technical assistance in the immunohistochemical staining and molecular procedures.
The authors have no conflicts of interest to declare.
REFERENCES
- Nazemi A, Higgins S, Swift R, In G, Miller K, Wysong A. Eccrine porocarcinoma: new insights and a systematic review of the literature. Dermatol Surg 2018; 44: 1247–1261.
- Salih AM, Kakamad FH, Baba HO, Salih RQ, Hawbash MR, Mohammed SH, et al. Porocarcinoma; presentation and management, a meta-analysis of 453 cases. Ann Med Surg (Lond) 2017; 20: 74–79.
- Sekine S, Kiyono T, Ryo E, Ogawa R, Wakai S, Ichikawa H, et al. Recurrent YAP1-MAML2 and YAP1-NUTM1 fusions in poroma and porocarcinoma. J Clin Invest 2019; 129: 3827–3832.
- Gómez-Zubiaur A, Medina-Montalvo S, Vélez-Velázquez MD, Polo-Rodríguez I. Eccrine porocarcinoma: patient characteristics, clinical and histopathologic features, and treatment in 7 cases. Actas Dermosifiliogr 2017; 108: e27–e32.
- Macagno N, Kervarrec T, Sohier P, Poirot B, Haffner A, Carlotti A, et al. NUT Is a specific immunohistochemical marker for the diagnosis of yap1-nutm1-rearranged cutaneous poroid neoplasms. Am J Surg Pathol 2021; 45: 1221–1227.
- Prieto-Granada C, Morlote D, Pavlidakey P, Rodriguez-Waitkus P, Ramirez C, Florento E, et al. Poroid adnexal skin tumors with YAP1 fusions exhibit similar histopathologic features: a series of six YAP1-rearranged adnexal skin tumors. J Cutan Pathol 2021; 48: 1139–1149.
- Parra O, Kerr DA, Bridge JA, Loehrer AP, Linos K. A case of YAP1 and NUTM1 rearranged porocarcinoma with corresponding immunohistochemical expression: review of recent advances in poroma and porocarcinoma pathogenesis with potential diagnostic utility. J Cutan Pathol 2021; 48: 95–101.
- Russell-Goldman E, Hornick JL, Hanna J. Utility of YAP1 and NUT immunohistochemistry in the diagnosis of porocarcinoma. J Cutan Pathol 2021; 48: 403–410.
- Godillot C, Boulinguez S, Riffaud L, Sibaud V, Chira C, Tournier E, et al. Complete response of a metastatic porocarcinoma treated with paclitaxel, cetuximab and radiotherapy. Eur J Cancer 2018; 90: 142–145.
- Belin E, Ezzedine K, Stanislas S, Lalanne N, Beylot-Barry M, Taieb A, et al. Factors in the surgical management of primary eccrine porocarcinoma: prognostic histological factors can guide the surgical procedure. Br J Dermatol 2011; 165: 985–989.
- Lee KA, Cioni M, Robson A, Bataille V. Metastatic porocarcinoma achieving complete radiological and clinical response with pembrolizumab. BMJ Case Rep 2019; 12: e228917.