SHORT COMMUNICATION
Acral Peeling Skin Syndrome: Two Unusual Cases and the Therapeutic Potential of Botulinum Toxin
Anna-Lotta STJERNBRANDT1, Magnus BURSTEDT2, Emma HOLMBOM3 and Alexander SHAYESTEH1*
Departments of 1Public Health and Clinical Medicine, Dermatology and Venereology, 2Medical Biosciences, Medical and Clinical Genetics, and 3Clinical Sciences, Obstetrics and Gynaecology, Umeå University, SE-901 85 Umeå, Sweden. *E-mail: alexander.shayesteh@umu.se
Citation: Acta Derm Venereol 2024; 104: adv24305. DOI https://doi.org/10.2340/actadv.v104.24305.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/)
Submitted: Oct 25, 2023; Accepted: Mar 19, 2024; Published: Apr 9, 2024
INTRODUCTION
Acral peeling skin syndrome (APSS) is a skin condition that causes painless peeling of the skin on hands and feet. APSS is described as being caused by an autosomal recessive mutation in the TGM5 (transglutaminase 5) gene and less frequently in the CSTA (cystatin A) gene (1, 2). Peeling in APSS usually starts at birth but it may also make its debut later in life. Common symptoms are itching, redness, erosions or sometimes blisters, and the skin heals without scarring (3). Humidity and friction are common exacerbating factors making APSS difficult to distinguish clinically from localised epidermolysis bullosa simplex (EBS) (4). Other common differential diagnoses are keratosis palmaris et plantaris and allergic contact dermatitis. As APSS has a relatively mild course, there is a considerable risk of underdiagnosis. In addition, because there is no cure for APPS, treatment is symptomatic by softening the skin and preventing friction.
CASE REPORT
Patient 1. A 23-year-old man was diagnosed with localised EBS by a paediatrician and a dermatologist at 1 year of age. Symptoms consisted of blisters, fissures, and pain when wearing socks or shoes. The patient was not treated until the age of 16 years when he consulted a paediatrician due to reddening, swelling, and pain in the penile skin after coitus. Physical examination revealed no skin changes and a culture for tinea was performed. He was referred to a dermatologist who diagnosed the skin problem as candida balanitis and treated it with hydrocortisone and miconazole cream without follow-up. The patient was referred to our department again at the age of 18, for a medical certificate exempting him from military service due to blisters and fragile skin on both the frontal and dorsal parts of his hands and feet (Fig. 1). Botulinum toxin type A (Btx-A) injections on the skin of palms and soles were suggested as a treatment option to reduce perspiration and ease his problems. A total of 5 sessions of Btx-A injections (Allergan, BOTOX; median dose 358 U [IQR 340-386]) on the frontal and dorsal sides of the palms and fingers, on both hands, was performed between 2018 and 2023. The patient abstained from treatment of the feet. The Btx-A treatments were described as helpful, primarily preventing the occurrence of blisters that would cause wounds and pain (Fig. 2). In 2023, the patient requested genetic counselling as he and his partner were in the process of parental planning and worried about the risks of genetic diseases in their offspring.
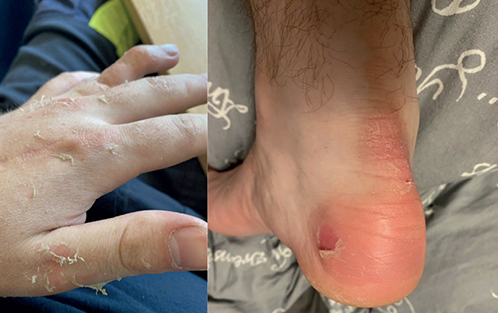
Fig. 1. Patient 1. Diffuse skin peeling without inflammation on the dorsal side of the palm, fingers, and back of the foot.
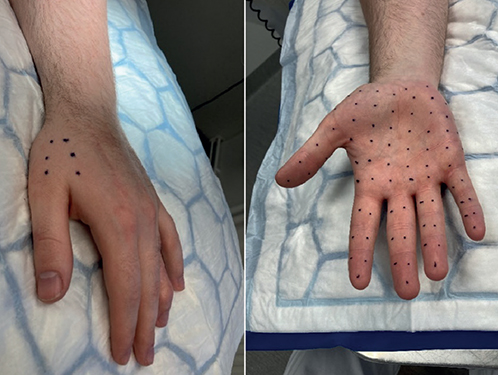
Fig. 2. Patient 1. No apparent skin peeling after repeated Btx-A injections on the frontal and dorsal sides of the palm.
Patient 2. A 51-year-old woman was diagnosed with localized epidermolysis bullosa simplex at our department at the age of 3 years. She suffered from blisters and vesicles, on the extensor and flexor parts of palms and soles, with exacerbation in the summer. At this age, a remedy of Vitamin E (di-alpha-tocopherol acetate) of 10 drops x 3 daily, in a total dose of 400 IE/daily, was prescribed but had no effect at the 3-month follow-up. At the age of 19 years, working at a grocery, the patient experienced recurring blisters on the skin of her palms and fingers due to increased friction. As her symptoms continued, she sought help from a dermatologist who diagnosed her as having an allergic contact dermatitis against Primula vulgaris and treated her with clobetasol propionate cream. No further contacts were made until 2023 as she was concerned about the offspring of her 2 children, then 18 and 20 years old, and wanted genetic counselling. She also described difficulties after delivery due to delayed healing of wounds and sutures in the skin, which had made her undergo a Caesarean for her second labour. Her children were asymptomatic, but she still complained about exacerbations of skin peeling and erosions on the skin of the distal extremities (Fig. 3).
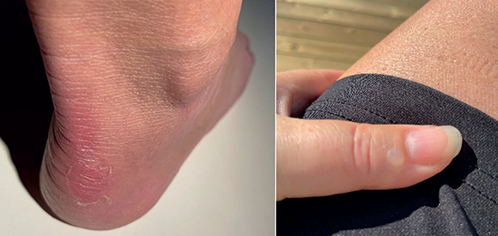
Fig. 3. Patient 2. Mild skin peeling on the back of the lower leg and a blister on the dorsal side of the thumb.
RESULTS
Genetic analysis of patients’ blood samples included a panel of 26 genes associated with EBS (Blueprint Genetics Epidermolysis Bullosa Panel (version 4, 19 October 2019, Plus Analysis). The analysis also included an examination of sequence gene variations as well as copy number analysis. The target region for each gene included coding exons and ± 20 base pairs from the exon–intron boundary. In addition, the panel using an Illumina NovaSeq 6000 platform, included non-coding and regulatory variants as well as clinically relevant non-coding variants. The genetic analysis showed that patients 1 and 2 were heterozygous for TGM5 c.337G>T, p.(Gly113Cys) and heterozygous for TGM5 c.763T>C, p.(Trp255Arg). No parental samples were available for analysis and the phase of the variants was not determined (Transcript: NM_201631.4 Assembly: GRCh37/hg19). Both variants found in our 2 patients were classified as pathogenic according to ACMG guidelines (5). The analysis also revealed a heterozygous variant in the EXPH5 gene associated with autosomal recessive epidermis bullosa (EXPH5 c.959C>A, p.(Ser320*) [Transcript: NM_015065.3 Assembly: GRCh37/hg19]) in patient 1. Although the phases of the variants were not determined for our cases, the result of the genetic analysis performed and the clinical picture were considered enough for diagnosis.
DISCUSSION
Distinguishing APSS from localised EBS is a clinical challenge. Healthcare professionals should be aware that patients with APSS may seek medical help with unspecified symptoms from other parts of the body, such as the genitals or the distal extremities, caused by skin friction. Hence, symptoms according to APPS may not always be limited to hands and feet. Patients with recurrent blisters and skin peeling on hands and feet, without a history of atopy, should be provided with genetic counselling and testing. The genetic investigation should include specimens for mutation analysis in TGM5/CSTA genes and mutations according to corresponding genes for localised EBS. The same combination of variants in the TGM5 gene was detected in our 2 unrelated patients. This indicates that the TGM5 c.763T>C, p.(Trp255Arg) mutation could be a founder variant, a pathogenic variant observed at high frequency due to the presence of the variant in a single ancestor or a small number of ancestors, in northern parts of Sweden (6). TGM5 c.337G>T, p.(Gly113Cys) has been previously detected in numerous individuals with acral peeling skin syndrome and is considered to be a founder variant in the European population. Of a total of 78 detected occurrences of TGM5 c.763T>C, p.(Trp255Arg) in the gnomAD v2.1.1 database, 49 were detected in the Swedish genetic ancestry group (7). Experiences from our 2 cases indicate that in APSS both frontal and dorsal sides of the skin on the hands and feet may be affected, while in localised EBS skin fragility is mainly located on the palms and soles (8). More case series are needed to confirm whether this finding is truly significant for the diagnosis of APSS.
While there is no curative treatment available for APSS, recommended topical or systemic keratolytic medications are seldom helpful. Hyperhidrosis is often described to exacerbate symptoms in APSS. To the best of our knowledge, this is the first description of successful treatment of APSS with Btx-A. The rationale for this treatment was due to its potent effect of reducing hyperhidrosis (9). Btx-A treatment has also been described to reduce symptoms of pain and blistering in localised EBS and pachyonychia congenita, sharing common clinical features with APSS (10). Thus, Btx-A treatments on the palms and feet could be a good option for patients with APSS when topical treatments are unsuccessful.
REFERENCES
- Cañueto J, Bueno E, Rodríguez-Diaz E, Vicente-Díaz MA, Álvarez-Cuesta CC, Gonzalvo-Rodríguez, et al. Acral peeling skin syndrome resulting from mutations in TGM5. J Eur Acad Dermatol Venereol 2016; 30: 477–480.
- Muttardi K, Nitoiu D, Kelsell DP, O’Toole EA, Batta K. Acral peeling skin syndrome associated with a novel CSTA gene mutation. Clin Exp Dermatol 2016; 41: 394–398.
- Acral peeling skin syndrome. Genetic and Rare Diseases Information Center. Updated February 2023 [accessed September 2023]. Available from: https://rarediseases.info.nih.gov/diseases/12863/acral-peeling-skin-syndrome.
- Kiritsi D, Cosgarea I, Franzke CW, Schumann H, Oji V, Kohlhase J, et al. Acral peeling skin syndrome with TGM5 gene mutations may resemble epidermolysis bullosa simplex in young individuals. J Invest Dermatol 2010; 130: 1741–1746.
- Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015; 17: 405–424.
- Vahlquist A, Tasanen K. Epidermolysis bullosa care in Scandinavia. Dermatol Clin 2010; 28: 425–427.
- Karczewski KJ, Francioli LC, Tiao G, Cummings BB, Alföldi J, Wang Q, et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 2020; 581: 434–443.
- Sprecher E. Epidermolysis bullosa simplex. Dermatol Clin 2010; 28: 23–32.
- Nawrocki S, Cha J. Botulinum toxIn: pharmacology and injectable administration for the treatment of primary hyperhidrosis. J Am Acad Dermatol 2020; 82: 969–979.
- Swartling C, Karlqvist M, Hymnelius K, Weis J, Vahlquist A. Botulinum toxin in the treatment of sweat-worsened foot problems in patients with epidermolysis bullosa simplex and pachyonychia congenita. Br J Dermatol 2010; 163: 1072–1076.