ORIGINAL REPORT
Filaggrin Mutation Status and Prevention of Atopic Dermatitis with Maternal Probiotic Supplementation
Dinastry Pramadita ZAKIUDIN1,2, Jacob P. THYSSEN3, Claus ZACHARIAE4,5, Vibeke VIDEM6,7, Torbjørn ØIEN1 and Melanie Rae SIMPSON1
1Department of Public Health and Nursing, NTNU – Norwegian University of Science and Technology, Trondheim, 2Clinic for Laboratory Medicine, St Olavs Hospital, Trondheim, Norway, 3Department of Dermatology and Venereology, Bispebjerg Hospital, University of Copenhagen, Copenhagen, 4Department of Clinical Medicine, University Hospital of Copenhagen Gentofte, Hellerup, 5Department of Dermatology and Allergy, University Hospital of Copenhagen Gentofte, Hellerup, Denmark, 6Department of Clinical and Molecular Medicine, NTNU – Norwegian University of Science and Technology, Trondheim, 7Department of Immunology and Transfusion Medicine, St Olavs Hospital, Trondheim University Hospital, Trondheim, Norway
Abstract
The World Allergy Organization recommends probiotics in the prevention of atopic dermatitis in high-risk populations. Mutations in the filaggrin gene (FLG) result in an increased risk of atopic dermatitis through disruption of the skin keratin layer. This exploratory study investigated whether the preventive effect of maternal probiotics was evident in children with and without FLG mutations. DNA was collected from children (n = 228) from the Probiotic in the Prevention of Allergy among Children in Trondheim (ProPACT) study. Samples were analysed for 3 common FLG mutations (R501X, R2447X, and 2282del4). Overall, 7% of children had heterozygous FLG mutations; each child had only one of the 3 mutations. Mutation status had no association with atopic dermatitis (RR = 1.1; 95% CI 0.5 to 2.3). The risk ratio (RR) for having atopic dermatitis following maternal probiotics was 0.6 (95% CI 0.4 to 0.9) and RR was similar if the child expressed an FLG mutation (RR = 0.6; 95% CI 0.1 to 4.1) or wildtype FLG (RR = 0.6; 95% CI 0.4 to 0.9). The preventive effect of probiotics for atopic dermatitis was also evident in children without FLG mutation. Larger confirmatory studies are needed.
SIGNIFICANCE
In our population study, maternal probiotic supplementation also appeared to be effective to prevent atopic dermatitis among those without filaggrin gene (FLG) mutations with a 40% reduced risk of contracting atopic dermatitis. This finding is in contrast to the World Allergy Organization’s recommendation of probiotic supplementation only to mothers and infants with high risk of allergy.
Key words: atopic dermatitis; child, preschool; filaggrin; gene; probiotic.
Citation: Acta Derm Venereol 2024; 104: adv24360. DOI https://doi.org/10.2340/actadv.v104.24360.
Copyright: © 2024 The Author(s). Published by MJS Publishing, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/).
Submitted: Oct 26, 2023; Accepted after revision: Mar 22, 2024; Published: Apr 24, 2024
Corr: Dinastry Pramadita Zakiudin, Department of Public Health and Nursing, NTNU – Norwegian University of Science and Technology, Håkon Jarls gate 11, NO-7030, Trondheim, Norway. E-mail: dinastry.p.zakiudin@ntnu.no
Competing interests and funding: The authors have no conflicts of interests to declare.
INTRODUCTION
Atopic dermatitis (AD) or eczema is the most common inflammatory skin disease in children, posing a significant burden (1, 2). Its pathogenesis involves barrier dysfunction, altered immune responses, impaired skin microbial biodiversity, and interaction between genetic inheritance and the environment (3, 4).
Mutations in the filaggrin gene (FLG), which codes for filament aggregating protein, have been described as a major risk factor for AD (5). Filaggrin is important in forming the keratin layer and for skin hydration (6). Mutations of FLG lead to decreased expression, thus increasing the risk of both AD and subsequent allergic sensitisation. The gene is located within the epidermal differentiation complex (EDC) region on chromosome 1q21 (7). Three mutations (R501X, R2447X, and 2282del4) are common in populations of Northern European descent (8, 9) and, together with S3247X, they account for more than 90% of the total mutations in FLG in European populations (8, 10, 11). These are loss-of-function mutations leading to truncation of filaggrin translation by creating premature termination codons (10, 12), and can result in epidermal barrier defects (13).
The skin and gut act as the immune system’s first line of defence facing allergens and pathogens (13), and environmental stimulation can affect immune responses at the epidermal barriers (14, 15). Alterations in the gut microbiome may result from environmental exposures and can influence immune responses and possibly be associated with abnormal epidermal barrier function in AD (13). Probiotics are live microorganisms that confer a health benefit when administered in adequate amounts (16). The World Allergy Organization (WAO) recommends probiotic supplementation for high-risk mothers and infants who are defined as having a biological parent or sibling with existing or a history of allergic rhinitis, asthma, eczema, or food allergy (17). This recommendation is based on several trials, which have found that probiotic supplementation may have a preventive effect on AD in infancy (18, 19). Increased transcription factors for FLG were also observed in mesenteric lymph nodes in mice after probiotic intervention (20). However, there are no studies exploring the role of FLG mutations as a genetic vulnerability to AD in relation to the preventive effect of probiotics (18, 21, 22).
In our randomised placebo-controlled study, Probiotics in the Prevention of Allergy among Children in Trondheim (ProPACT), short-term administration of probiotic bacteria given to a non-selected population of pregnant women reduced the cumulative incidence of AD in their offspring by 40% at 2 years of age (19). Given that we observed the greatest preventive effect among children without a family history of atopy (19), we hypothesised that children with FLG mutations would benefit less from probiotic supplementation. Our primary aim was to undertake an exploratory analysis of whether the preventive effect of maternal probiotics was evident in children both with and without FLG mutations. As secondary aims, we explored whether FLG mutations were associated with AD severity, allergic sensitisation, wheezing episodes, and family history of atopy and AD.
MATERIALS AND METHODS
Participants and sample collection
The ProPACT study followed 415 pregnant women randomised to receive probiotic or placebo milk from 36 weeks of gestation until 3 months post-delivery while breastfeeding (19, 23). The pregnant women were recruited from a non-selected population, and a computer-generated randomisation sequence allocated them to probiotic or placebo milk. The probiotic milk corresponded to a daily dose of 5x1010 colony-forming units (CFU) of Lactobacillus rhamnosus GG (LGG), 5x1010 CFU of Bifidobacterium animalis subsp. lactis Bb-12 (Bb-12), and 5x109 CFU of Lactobacillus acidophilus La-5 (La-5), whilst the placebo milk was fermented and pasteurised skimmed milk with similar taste but without probiotic bacteria. Information regarding demographics and risk factors for allergy-related diseases was obtained from questionnaires completed during pregnancy, and at the ages of 6 weeks, 1 year, and 2 years. Children were encouraged to attend an examination by a trained nurse if they had an itchy rash for more than 4 weeks any time during the first year of life to ensure all AD cases were identified. At 2 years of age, a paediatrician examined all children and AD was defined using the UK working party’s diagnostic criteria for AD (24). The AD severity was assessed with the Nottingham Eczema Severity Score (NESS) (25). Allergic sensitisation was examined through a skin prick test (SPT) or elevated specific IgE (≥ 0.35 kU L–1) (19). Wheezing that could develop into asthma later in life was defined as at least 3 episodes of wheezing in the last 12 months combined with treatment by inhaled glucocorticoids, or signs of suspected hyper-reactivity (cough or wheeze on excitement or impaired night sleep) without concurrent upper respiratory infection.
Children were eligible for inclusion in this study if they had provided a blood sample at either 2 or 6 years of age and had attended the clinical examination at 2 years. Ultimately, 228 children were included, 111 from the probiotic group and 117 from the placebo group (Fig. 1). All participating families signed written consent. The study was approved by the Regional Committee for Medical Research Ethics in Central Norway (097-03) and registered at ClinicalTrials.gov (NCT00159523).
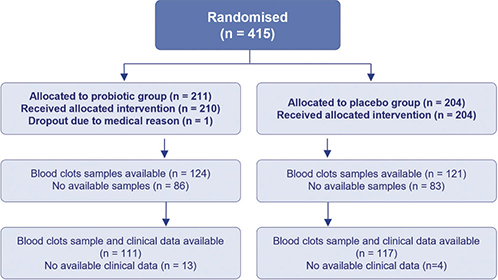
Fig. 1. Inclusion of the participants. Flow of subjects in the probiotic and placebo groups.
Filaggrin gene (FLG) analyses
Genomic DNA from blood clots was extracted from blood samples collected between February 2006 and December 2011 from children at 2 or 6 years and stored at –80°C until analysis, which was completed in February 2023. Thawed samples underwent homogenisation before DNA isolation, which was done from leukocytes using the MasterPure DNA purification kit (Lucigen, Middleton, WI, USA) according to the manufacturer’s instructions. DNA isolates were then sequenced using a TaqMan genotyping assay for the sites of R501X, R2447X, and 2282del4 on the FLG gene as previously described (26). The analysis can provide 3 different qualitative analysis results: wildtype (WT) as the reference with no present mutation, heterozygous for mutation, or homozygous for mutation.
Analysis DNA by KBA-HGH (Klinisk Biokemisk Afdeling Herlev og Gentofte Hospital) Denmark
The analysis principle is allelic discrimination using TaqMan probes:
- Primers (short sequence of nucleotides) bind to 3 specific sites on the filaggrin gene (FLG), which include the genomic locations for the mutations R501X, R2447X, and 2282del4. The primers facilitate amplification of the 3 genomic locations.
- Six TaqMan probes, which are specific for R501X wild type, R501X mutant, R2447X wild type, R2447X mutant, 2282del4 wild type, and 2282del4 mutant, bind to the amplified DNA and emit a fluorescence signal upon binding.
Precision control materials use previously analysed patient samples whose genotypes have been confirmed by analysis with an alternative laboratory method (Sanger sequencing) and control levels use 8 controls: wild type, heterozygous, and homozygous for the R501X and 2282del4 assays and wild type and heterozygous for the R2447X assay.
Statistical analyses
The statistical analyses were performed using Stata/IC 17 (StataCorp, College Station, TX, USA). Descriptive variables are presented as mean (standard deviation, SD) for continuous variables, and frequency (percentage) for categorical variables. We compared the proportion of FLG mutations and the risk ratio (RR) of having an AD diagnosis in each group (probiotic and placebo groups) to investigate the role of FLG in probiotic treatment to prevent AD. We analysed differences in proportions having several characteristics of AD in each group of mutation and WT by comparing odds ratio (OR) using univariate logistic regression.
RESULTS
A total of 228 blood samples were included (Fig. 1). In this subgroup from the ProPACT study, the probiotic group had a higher proportion of male infants (50% vs 41%), and older siblings (44% vs 38%) compared with the placebo group (Table I). There were also fewer with a maternal history of atopy in the probiotic group (47% vs 56%), although the proportion with a family history was similar (65% vs 70%) (Table I). These findings were comparable to the overall ProPACT study (19, 23). Also consistent with the overall results from the ProPACT study (19, 23), the probiotic group in this study had a lower cumulative incidence of AD at 2 years of age (20% vs 34%, RR = 0.6; 95% confidence interval [CI] 0.4 to 0.9).
Our exploratory study observed that 7% of all the children had an FLG mutation. In all cases, these children had only one of the three heterozygous FLG mutations (R501X, 2282del4, R2447X). The FLG mutations were twice as common in the placebo group (Table I). There was no conclusive association between the presence of FLG mutation and AD (RR = 1.1; 95% CI 0.5 to 2.3) (Table II). The prevalence of AD was similar for children with and without an FLG mutation within the probiotic (20.0% and 19.8%, respectively) and within the placebo group (33.3% and 34.3%). Therefore, the presence of FLG mutations did not appear to modify the preventive effect from maternal probiotics, although we could not exclude a possible influence of the mutation based on the wide range of the CI. Specifically, the RR of having AD following maternal probiotic supplementation was 0.6 (95% CI 0.1 to 4.1) for children with FLG mutations and 0.6 (95% CI 0.4 to 0.9) for those with WT.
| AD at 2 years | Any mutationa (n = 17) | Wildtype (n = 211) | Total (n = 228) | |||
| Without atopic dermatitis | 12 (71%) | 154 (73%) | 166 (73%) | |||
| Atopic dermatitis | 5 (29%) | 57 (27%) | 62 (29%) | |||
| Probiotic | Placebo | Probiotic | Placebo | Probiotic | Placebo | |
| Without atopic dermatitis | 4 (80%) | 8 (66.7%) | 85 (80.2%) | 69 (65.7%) | 89 (80.2%) | 77 (65.8%) |
| Atopic dermatitis | 1 (20%) | 4 (33.3%) | 21 (19.8%) | 36 (34.3%) | 22 (19.8%) | 40 (34.2%) |
| Total | 5 | 12 | 106 | 105 | 111 | 117 |
| aAny mutation of R501X, 2282del4, and R2447X (n = 17). | ||||||
When exploring several characteristics of AD, there were too few children with AD and FLG mutations to consider differences in proportions in some AD characteristics. There were few cases of FLG mutations with AD in our population (n = 5) to compare FLG mutations and WT on each characteristic such as allergic sensitisation (n = 2), wheezing (n = 1), or severity (2 cases with FLG mutations had mild and 3 cases with FLG mutations had moderate–severe). Among 17 children with FLG mutations there were 13 children (76%) with atopy history in the family, of whom 10 children (59%) had a family history of AD. All children with a single FLG mutation developed AD before 6 months of age, which was a higher proportion compared with WT (30% vs 16%) (Table III).
DISCUSSION
wIn this study, we undertook an exploratory analysis of the role of FLG mutations in the prevention of AD following maternal probiotic supplementation. We observed no clear difference in the preventive effect of maternal probiotics between children with or without one of the examined FLG mutations. That is to say, the preventive effect seen in the main study was also present among the children without any of the analysed FLG mutations. However, there were only a few children with an FLG mutation, and we cannot exclude the possibility that the presence of FLG mutation results in greater or lesser prevention of AD following maternal probiotic supplementation. FLG mutations were otherwise more common in children diagnosed with AD at a young age (less than 6 months of age). There were too few children with FLG mutations who had AD to consider comparison in other subgroups and our prevalence of FLG mutations was slightly lower than the Scandinavian infants’ cohort (7% vs 9%) (27).
The prevention of AD following probiotic supplementation around the time of birth has been observed previously (19, 23, 28). While our study aimed to explore whether children carrying FLG mutations had a lesser benefit from maternal probiotics, most of the children, both with and without AD, did not carry any of the FLG mutations. We also observed no conclusive difference in the preventive effect between the children based on FLG mutation status. Importantly, we found that the preventive effect seen in the main study is preserved among the children without any of the analysed FLG mutations. Previous analyses of the ProPACT study suggest that the greatest preventive effect was seen in children without a family history of atopy (19), and together with the present results this indicates that maternal probiotic supplementation may also be beneficial for AD prevention in children not considered at “high risk” of developing AD. Contrastingly, it is for these “high-risk” infants that the WAO currently recommends probiotic supplementation for the mother and infant, with a low level of evidence (17). This recommendation reflects the fact that most studies have been conducted on “high-risk” populations, which the WAO defined as having a biological parent or sibling with existing or a history of allergic rhinitis, asthma, eczema, or food allergy (17). There is a need for more studies based on non-selected populations as used in the ProPACT study.
Given that probiotic supplementation also appears to be effective among those without FLG mutation, there may be other factors or interactions between the genes and the environment that contribute to the homeostasis of the skin barrier following maternal probiotic supplementation. Whilst probiotics given to mothers is one of the proposed environmental exposures that influences the epigenome of the child (29, 30), there is a lack of direct data on the impact of consumed probiotics on the epigenetic process in allergy (29). Maternal probiotics in an animal model did not alter FLG expression in their offspring (22), although another animal study observed increased transcription of FLG after direct probiotic supplementation (20). There is no other study to date that has explored the interaction between FLG mutation and probiotic exposure either directly given to infants or provided as maternal supplementation.
All children in this study had only one type of FLG mutation and were heterozygous carriers of the mutation. Although a previous study observed that heterozygous carriers showed no significant increase in risk in AD (31), others have described that filaggrin deficiency still can manifest following loss-of-function mutations on a single allele, a situation known as haploinsufficiency (32). It has been suggested that this haploinsufficiency can result in a skin phenotype of AD (32), typically with a milder severity (5). This may have been the case for the children in our study, who mostly had mild severity of disease. However, the prevalence of FLG mutation was low, and we found no clear association between the presence of FLG mutation and AD.
When considering other characteristics of AD, we did not have sufficient numbers to explore any difference in proportions of several characteristics of AD based on the presence of FLG mutations or analysis in further subgroups. In a previous study, early-onset AD was observed to be affected more by genetic factors, whereas environmental exposures, such as antibiotics and probiotic consumption, appeared to have a greater influence on late-onset AD (33). This is in line with our observation that FLG mutation was more common in those with symptom debut before 6 months, but our estimates are exploratory findings and should be interpreted cautiously.
Strength and limitations
A key strength of this study is the double-blinded randomised design of the probiotic intervention and the novel investigation of the interplay between FLG mutation and probiotics in AD prevention (22). Furthermore, the participants showed high compliance with the intervention, consumed only study milks, and did not report taking additional probiotic products during the study period. AD was diagnosed using detailed clinical information based on validated criteria. Families were encouraged to attend the dermatology clinic if their children developed an itchy rash lasting more than 4 weeks to capture all cases of AD. Whilst this was part of the study protocol for children up to 1 year of age, many families took this opportunity up to 20 months of age. We therefore think it unlikely that any cases of AD were undiagnosed, although theoretically would be possible if the symptoms presented and resolved between 1 and 2 years of age.
The major limitation of this study is that the number of participants with FLG mutations was small. The previously described relationship between FLG mutations and early-age AD diagnosis (34) could not be shown conclusively in this study. Furthermore, most of the children had mild AD, which made it difficult to investigate the relation of severity with FLG mutation as previous studies have suggested (35, 36). The results should be interpreted cautiously and need to be confirmed in larger studies.
Conclusion
Our study was too small to determine conclusively whether children with FLG mutations benefit from maternal probiotic supplementation in the prevention of AD. However, we found that the previously reported preventive effect was present in the children without FLG mutation. As FLG mutation was found to be relatively rare in this study, and the preventive effect was evident in those without FLG mutation, our study supports the theory that maternal probiotics are also beneficial for AD prevention in children without FLG mutation. The impact of probiotic consumption as an environmental factor in allergy development remains understudied, and further studies are warranted, particularly in non-selected populations.
ACKNOWLEDGEMENTS
The authors would like to thank the parents and the participants in the study, the project assistants Guri Helmersen and Else Bartnes, the midwives of the Trondheim region for their help in recruitment, and paediatrician Rakel Berg who examined the children.
Funding sources: The Liaison Committee between the Central Norway Regional Health Authority and NTNU – Norwegian University of Science and Technology, and the Norwegian Research Council (grant reference number 2019/38881). Tine BA sponsored the study through supply and distribution of the study milk. The funding sources had no role in the study design, data collection, analysis, interpretation of the results, or writing of the manuscript.
IRB approval status: The study was approved by the Regional Committee for Medical Research Ethics in Central Norway (097-03).
REFERENCES
- McKenna SP, Doward LC, Meads DM, Tennant A, Lawton G, Grueger J. Quality of life in infants and children with atopic dermatitis: addressing issues of differential item functioning across countries in multinational clinical trials. Health Qual Life Outcomes 2007; 5: 45.
- Williams HC. Clinical practice. Atopic dermatitis. N Engl J Med 2005; 352: 2314–2324.
- Huang YJ, Marsland BJ, Bunyavanich S, O’Mahony L, Leung DY, Muraro A, et al. The microbiome in allergic disease: current understanding and future opportunities – 2017 PRACTALL document of the American Academy of Allergy, Asthma & Immunology and the European Academy of Allergy and Clinical Immunology. J Allergy Clin Immunol 2017; 139: 1099–1110.
- Dong TS, Gupta A. Influence of early life, diet, and the environment on the microbiome. Clin Gastroenterol Hepatol 2019; 17: 231–242.
- Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet 2006; 38: 441–446.
- Hoste E, Kemperman P, Devos M, Denecker G, Kezic S, Yau N, et al. Caspase-14 is required for filaggrin degradation to natural moisturizing factors in the skin. J Invest Dermatol 2011; 131: 2233–2241.
- Cookson WO, Ubhi B, Lawrence R, Abecasis GR, Walley AJ, Cox HE, et al. Genetic linkage of childhood atopic dermatitis to psoriasis susceptibility loci. Nat Genet 2001; 27: 372–373.
- Irvine AD, McLean WH, Leung DY. Filaggrin mutations associated with skin and allergic diseases. N Engl J Med 2011; 365: 1315–1327.
- Gupta J, Margolis DJ. Filaggrin gene mutations with special reference to atopic dermatitis. Curr Treat Options Allergy 2020; 7: 403–413.
- Sandilands A, Terron-Kwiatkowski A, Hull PR, O’Regan GM, Clayton TH, Watson RM, et al. Comprehensive analysis of the gene encoding filaggrin uncovers prevalent and rare mutations in ichthyosis vulgaris and atopic eczema. Nat Genet 2007; 39: 650–654.
- Brown SJ, Elias MS, Bradley M. Genetics in atopic dermatitis: historical perspective and future prospects. Acta Derm Venereol 2020; 100: adv00163.
- Smith FJ, Irvine AD, Terron-Kwiatkowski A, Sandilands A, Campbell LE, Zhao Y, et al. Loss-of-function mutations in the gene encoding filaggrin cause ichthyosis vulgaris. Nat Genet 2006; 38: 337–342.
- Fang Z, Li L, Zhang H, Zhao J, Lu W, Chen W. Gut microbiota, probiotics, and their interactions in prevention and treatment of atopic dermatitis: a review. Front Immunol 2021; 12: 720393.
- Rigoni R, Fontana E, Dobbs K, Marrella V, Taverniti V, Maina V, et al. Cutaneous barrier leakage and gut inflammation drive skin disease in Omenn syndrome. J Allergy Clin Immunol 2020; 146: 1165–1179 e11.
- Parodi A, Paolino S, Greco A, Drago F, Mansi C, Rebora A, et al. Small intestinal bacterial overgrowth in rosacea: clinical effectiveness of its eradication. Clin Gastroenterol Hepatol 2008; 6: 759–764.
- FAO/WHO. Evaluation of health and nutritional properties of powder milk and live lactic acid bacteria. Joint FAO/WHO Expert Consultation Cordoba, Argentina, 2001: p. 2.
- Fiocchi A, Pawankar R, Cuello-Garcia C, Ahn K, Al-Hammadi S, Agarwal A, et al. World Allergy Organization–McMaster University Guidelines for Allergic Disease Prevention (GLAD-P): Probiotics. World Allergy Organ J 2015; 8: 4.
- Cao L, Wang L, Yang L, Tao S, Xia R, Fan W. Long-term effect of early-life supplementation with probiotics on preventing atopic dermatitis: a meta-analysis. J Dermatolog Treat 2015; 26: 537–540.
- Dotterud CK, Storro O, Johnsen R, Oien T. Probiotics in pregnant women to prevent allergic disease: a randomized, double-blind trial. Br J Dermatol 2010; 163: 616–623.
- Kim IS, Lee SH, Kwon YM, Adhikari B, Kim JA, Yu DY, et al. Oral administration of beta-glucan and lactobacillus plantarum alleviates atopic dermatitis-like symptoms. J Microbiol Biotechnol 2019; 29: 1693–1706.
- D’Elios S, Trambusti I, Verduci E, Ferrante G, Rosati S, Marseglia GL, et al. Probiotics in the prevention and treatment of atopic dermatitis. Pediatr Allergy Immunol 2020; 31: 43–45.
- Marsella R, Santoro D, Ahrens K, Thomas AL. Investigation of the effect of probiotic exposure on filaggrin expression in an experimental model of canine atopic dermatitis. Vet Dermatol 2013; 24: 260–e57.
- Simpson MR, Dotterud CK, Storrø O, Johnsen R, Øien T. Perinatal probiotic supplementation in the prevention of allergy related disease: 6 year follow up of a randomised controlled trial. BMC Dermatol 2015; 15: 1–8.
- Williams HC, Burney PG, Hay RJ, Archer CB, Shipley MJ, Hunter JJ, et al. The U.K. Working Party’s Diagnostic Criteria for Atopic Dermatitis. I. Derivation of a minimum set of discriminators for atopic dermatitis. Br J Dermatol 1994; 131: 383–396.
- Emerson RM, Charman CR, Williams HC. The Nottingham Eczema Severity Score: preliminary refinement of the Rajka and Langeland grading. Br J Dermatol 2000; 142: 288–297.
- Meldgaard M, Szecsi PB, Carlsen BC, Thyssen JP, Johansen JD, Menne T, et al. A novel multiplex analysis of filaggrin polymorphisms: a universally applicable method for genotyping. Clin Chim Acta 2012; 413: 1488–1492.
- Hoyer A, Rehbinder EM, Fardig M, Asad S, Lodrup Carlsen KC, Endre KMA, et al. Filaggrin mutations in relation to skin barrier and atopic dermatitis in early infancy. Br J Dermatol 2022; 186: 544–552.
- Sun S, Chang G, Zhang L. The prevention effect of probiotics against eczema in children: an update systematic review and meta-analysis. J Dermatolog Treat 2021; 33: 1844–1854.
- Nedoszytko B, Reszka E, Gutowska-Owsiak D, Trzeciak M, Lange M, Jarczak J, et al. Genetic and epigenetic aspects of atopic dermatitis. Int J Mol Sci 2020; 21: 1–18.
- Forsberg A, Huoman J, Soderholm S, Bhai Mehta R, Nilsson L, Abrahamsson TR, et al. Pre- and postnatal lactobacillus reuteri treatment alters DNA methylation of infant T helper cells. Pediatr Allergy Immunol 2020; 31: 544–553.
- Brown SJ, Relton CL, Liao H, Zhao Y, Sandilands A, Wilson IJ, et al. Filaggrin null mutations and childhood atopic eczema: a population-based case-control study. J Allergy Clin Immunol 2008; 121: 940–946.e3.
- Brown SJ, Relton CL, Liao H, Zhao Y, Sandilands A, McLean WH, et al. Filaggrin haploinsufficiency is highly penetrant and is associated with increased severity of eczema: further delineation of the skin phenotype in a prospective epidemiological study of 792 school children. Br J Dermatol 2009; 161: 884–889.
- Loo EX, Shek LP, Goh A, Teoh OH, Chan YH, Soh SE, et al. Atopic dermatitis in early life: evidence for at least three phenotypes? Results from the GUSTO Study. Int Arch Allergy Immunol 2015; 166: 273–279.
- Smieszek SP, Welsh S, Xiao C, Wang J, Polymeropoulos C, Birznieks G, et al. Correlation of age-of-onset of atopic dermatitis with filaggrin loss-of-function variant status. Sci Rep 2020; 10: 2721.
- Kezic S, O’Regan GM, Yau N, Sandilands A, Chen H, Campbell LE, et al. Levels of filaggrin degradation products are influenced by both filaggrin genotype and atopic dermatitis severity. Allergy 2011; 66: 934–940.
- O’Regan GM, Kemperman PM, Sandilands A, Chen H, Campbell LE, Kroboth K, et al. Raman profiles of the stratum corneum define 3 filaggrin genotype-determined atopic dermatitis endophenotypes. J Allergy Clin Immunol 2010; 126: 574–580 e571.