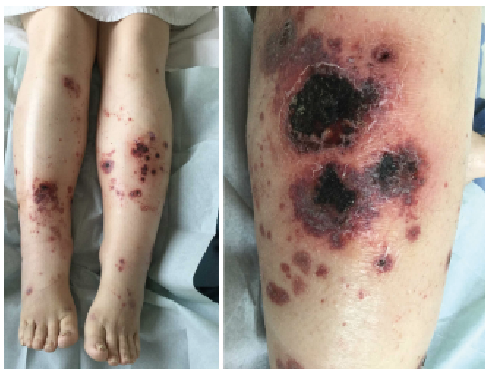
Fig. 1. Lower-limb purpura 3 months after onset of symptoms.
Departments of 1Dermatology-Venereology and 4Gastrology, Robert-Debré Hospital, Avenue du Général Koenig, FR-51092 Reims, 2Biopathology and 3Department of Rheumatology, Maison-Blanche Hospital, Reims University Hospital, Reims, France. *E-mail: mviguier@chu-reims.fr
Accepted Feb 19, 2020; Epub ahead of print Feb 28, 2020
Acta Derm Venereol 2020; 100: adv00077
In the past few years, psoriasis and psoriatic arthritis (PsA) treatment has undergone a revolution, with the approval of biotherapies targeting tumour necrosis factor α (TNFα), interleukin (IL)-12/IL-23, IL-17 or IL-23. The safety profile of TNFα and anti-IL-12/IL-23 is now well known (1) and, in particular, cutaneous adverse events (AEs) are well identified (2). Recently, IL-17 inhibitors were introduced for psoriasis and PsA. They placed patients at risk of cutaneous and mucosal Candida infections (3), but little is known about other specific cutaneous AEs. Although previously reported with anti-TNFα, cutaneous vasculitis has rarely been reported during IL-17 blockage. We report here a case of severe cutaneous vasculitis with gut involvement after treatment with secukinumab for PsA.
A 54-year-old woman who had axial and peripheral PsA for 6 years was referred to our dermatology unit for cutaneous purpura progressing for 3 months, recently associated with febrile diarrhoea. Previous treatments for PsA included methotrexate alone, then methotrexate plus adalimumab. After one year of treatment with adalimumab and methotrexate, she had developed paradoxical pustular palmoplantar psoriasis, which was controlled by topical steroids. Two years later, her PsA was no longer controlled and adalimumab was switched to secukinumab, in association with methotrexate, with rapid control of PsA. One month after starting secukinumab, the patient developed psoriasiform lesions on the vulva, with nodular and pustular purpura on the lower limbs. Secukinumab was stopped 2 months after onset. One month later, the patient presented sub-acute febrile diarrhoea with persisting necrotic purpura on the lower limbs (Fig. 1). Biological analyses showed normal platelet count, renal and liver function, elevated C-reactive protein level, and negative serology for HIV or hepatitis B or C viruses. Search for cryoglobulinaemia, rheumatoid factor, anti-double-stranded DNA and anti-neutrophil cytoplasmic antibodies was negative. Anti-nuclear antibodies were positive, at 1:100 titres, with nuclear speckled pattern; C4 complement fraction was low. No proteinuria was detected. A faecal microbial search was negative and the faecal calprotectin rate was not specific (118 µg/l). Abdominal computed tomography revealed diffuse recto-pancolitis with terminal ileitis, without abscess or fistula (Fig. 2). Magnetic resonance imaging revealed pancolitis only. A short colonoscopy revealed purpuric inflammatory mucous with aphthoid ulcerations. Digestive biopsies suggested chronic ischaemia, with a unique non-specific epithelioid granuloma. Skin biopsy revealed parakeratosis, acanthosis, lymphocytic and polynuclear perivascular dermal infiltrates, fibrinoid necrosis in blood vessels walls, leukocytoclasia and thrombosis. Direct immunofluorescence of skin samples showed C3 deposits in blood vessel walls, with no IgA deposits (Fig. S1).

Fig. 1. Lower-limb purpura 3 months after onset of symptoms.
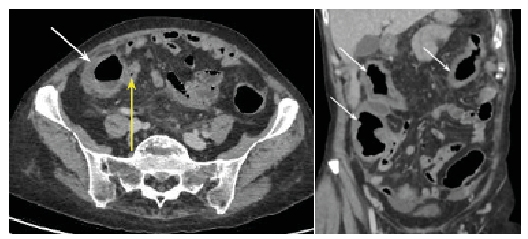
Fig. 2. Abdominal computed tomography showing pancolitis (white arrows), and terminal ileitis (yellow arrow).
Diagnosis of secukinumab-induced cutaneous and digestive vasculitis was suspected, and prednisone 1 mg/kg/day was started. The diarrhoea stopped rapidly and the skin lesions were attenuated. Digestive pan-endoscopy, performed one month later, revealed erythematous congestive rectocolic mucosa. Iterative biopsies were normal. Corticosteroids were gradually tapered over 7 weeks. A new flare of cutaneous purpura occurred within 1 week after withdrawal of steroids. A new regimen of prednisone, 0.5 mg/kg/day, associated with colchicine, 1 mg/day, was started. Partial remission, with only residual skin erosions, was obtained after 1 month, allowing a slow tapering of steroids within 6 months, while colchicine was maintained. Regarding PsA treatment, IL-17 blockers were contra-indicated and methotrexate was re-started at 15 mg/week. At 2 months after steroid withdrawal, there was no recurrence of cutaneous, digestive or joint symptoms.
Psoriasis and PsA are chronic inflammatory diseases, with a potentially high impact on quality of life (4). Inflammatory factors, as TNFα and IL-17, are implicated in their pathogenesis (5), and new therapies targeting IL-17 or their receptors have been developed. Secukinumab was the first anti-IL-17A therapy approved for psoriasis and PsA. Although it has shown high-sustained efficacy for both diseases (6, 7), this was not the case in Crohn’s disease (CD), which showed potential worsening (8).
The AEs most frequently reported with secukinumab are rhinopharyngitis, headache, and upper respiratory tract infections (3). Mucocutaneous Candida infections have been reported. Dysimmunological cutaneous reactions are widely described with anti-TNFα (psoriasiform lesions, cutaneous vasculitis, granulomatous dermatitis, rheumatoid nodes) (1), and similar cases with IL-17 blockage have been described. Atopic-like and eyelid dermatitis, psoriasiform eruption, granuloma annulare and drug-induced lupus erythematosus have been reported with secukinumab or ixekizumab (9–11).
A case of vasculitis triggered by secukinumab was described recently in a 28-year-old woman presenting cutaneous leukocytoclastic vasculitis after 8 months of secukinumab treatment for PsA (12). Secukinumab was stopped, and prednisone and cyclosporine controlled both the vasculitis and PsA. In the French pharmacovigilance database, we found 4 similar cases of cutaneous vasculitis, all in patients receiving secukinumab: 2 cases of cutaneous leukocytoclastic vasculitis in a 47-year-old man and a 58-year-old woman with PsA (after 2 months and 34 days of treatment respectively), one case of Henoch- Schönlein purpura after one month in a 42-year-old man with psoriasis, and one case of lower-limb purpura with unclear pathological findings in a 62-year-old woman with PsA after one month of treatment.
In the current case, the diagnosis of secukinumab-induced cutaneous and digestive vasculitis was suspected. Although cutaneous purpura developed soon after secukinumab onset, it did not initially improve after 4 weeks of drug discontinuation. Moreover, digestive symptoms, eventually related to gut involvement by vasculitis, appeared during this period. This apparently paradoxical evolution can be explained by the long mean half-life of secukinumab, which is 27 days. The major digestive differential diagnosis here would have been the induction or unveiling by secukinumab of latent CD. Indeed, and despite the absence of a history of CD, the previous treatment with anti-TNFα could have controlled the symptoms of an underlying digestive disease. Along the same lines, the patient’s inflammatory rheumatism, identified as PsA, was mainly axial and without cutaneous psoriasis lesions; thus, she may have had ankylosing spondylitis associated with CD rather than PsA.
The pathophysiology of vasculitis after secukinumab is unclear, and could implicate cytokine imbalance or immune complexes, as hypothesized in anti-TNFα-induced vasculitis (13). Anti-TNFα drugs may enhance T-helper 17 lymphocyte (Th17) numbers, leading to increased production of IL-22 (14). This situation creates a favourable immunological context that is conducive to AEs such as vasculitis. Thus, blockade of IL-23 with ustekinumab or guselkumab may be an interesting alternative therapy, as IL-23 is known to be the main driver and sustainer of Th17 differentiation. Further studies are needed to assess whether similar pathways are involved in the pathophysiology of anti-IL-17 AEs.
We report here the first case of secukinumab-induced vasculitis with cutaneous and gut involvement.
We are indebted to Professor Christine Hoeffel (Radiology Department, University Hospital of Reims) for radiological analysis.
Conflicts of interest: CC received a grant from Sanofi. JL received fees from Abbvie. MV has been an investigator, a board member or has received fees from Amgen, Boehringer, Novartis, Leo Pharma, Lilly, Arrow, Abbvie, Janssen, MSD, Pfizer, or Medac. All other authors have no conflict of interest to declare.