ORIGINAL ARTICLE
Measuring Quality of Life in Hidradenitis Suppurativa: Development and Validation of a Disease-specific Patient-reported Outcome Measure for Practice and Research
Marina OTTEN1, Matthias AUGUSTIN1, Christine BLOME1, Janine TOPP1, Marina NIKLAUS1, Caroline HILBRING1, Falk G. BECHARA2, Andreas PINTER3, Christos C. ZOUBOULIS4, Florian ANZENGRUBER5 and Natalia KIRSTEN1
1Institute for Health Services Research in Dermatology and Nursing (IVDP), University Medical Center Hamburg-Eppendorf (UKE), Hamburg, 2Dermatologic Surgery Unit, Department of Dermatology, Ruhr-University Bochum, Bochum, 3Clinic for Dermatology, Venereology and Allergology, University Hospital Frankfurt, Frankfurt am Main, 4Department of Dermatology, Venereology, Allergology and Immunology, Dessau Medical Center, Dessau, Germany, and 5Department of Dermatology, University Hospital Zurich, Zurich, Switzerland
Hidradenitis suppurativa is a chronic disease that disrupts patients’ physical and psychological well-being. A disease-specific measure was developed and validated for assessing health-related quality of life in hidradenitis suppurativa. After qualitative item development, the quality of life in hidradenitis suppurativa instrument was tested in 101 patients, applying convergent measures and a usability questionnaire. Descriptive and validation-specific analyses were conducted. There was no ceiling, but moderate floor effects (scores between 0 and 3.13 on a scale of 0–4). Few missing values were observed (21 of 23 items < 5%). Internal consistency was satisfying: 2 subscales with 6 and 16 items were identified (Cronbach’s alpha=0.95 and 0.88). The quality of life in hidradenitis suppurativa instrument correlated significantly with all convergent criteria (including change in convergent patient-reported outcomes; p < 0.05) except for Hurley stage (p = 0.490). In conclusion, the quality of life in hidradenitis suppurativa questionnaire is an internally consistent, valid, responsive, and usable instrument to assess quality of life in patients with hidradenitis suppurativa.
Key words: quality of life; burden; well-being; patient-reported outcome measure; validation; hidradenitis suppurativa.
Citation: Acta Derm Venereol 2023; 103: adv00859. DOI https://doi.org/10.2340/actadv.v102.2485.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Sep 21, 2022; Epub ahead of print: Sep 21, 2022; Published: Jan 31, 2023
Corr: Marina Otten, Institute for Health Services Research in Dermatology and Nursing, University Medical Center Hamburg-Eppendorf, Martinistraße 52, DE-20251 Hamburg, Germany. E-mail: m.otten@uke.de
Competing interests and funding: The authors have no conflicts of interest to declare.
Hidradenitis suppurativa (HS) is a chronic, inflammatory, recurrent, and debilitating skin disease of the hair follicle. It usually presents after puberty with painful, deep-seated, inflamed lesions in the apocrine gland-bearing areas of the body, most commonly the axillae, inguinal and anogenital regions (1, 2). It is characterized by active nodules, abscesses, and tunnels (3, 4), which may rupture externally and result in the secretion of a foul-smelling, purulent discharge (3). Pain due to the skin lesions can restrict everyday tasks and leads to frequent incapacity to work, or even occupational disability (5). The overall prevalence of HS has been calculated at approximately 0.4% (6), varying widely between 0.1% and 4.1%, depending on the methodological approach of the study (7–11). HS mainly remains poorly understood and managed. Even with a better understanding of pathogenesis, the aetiology of HS is not fully understood (12–14).
SIGNIFICANCE
Hidradenitis suppurativa is a chronic, inflammatory, recurrent, and debilitating skin disease with painful, deep-seated, inflamed lesions. It has a high impact on patients’ quality of life, which suggests measuring quality of life in clinical studies and clinical practice. A specific quality of life instrument was developed and validated, which can be used in both contexts and thus can support improving patient care.
Patients with HS are also at significantly higher risk of depression (5.9% vs 3.5% in controls) or anxiety disorders (3.9% vs 22.4% in controls), which are also significantly after controlling for age and sex (15). In general, 29.4% of patients with HS show additional mental health symptoms, mainly depression, but also, for example, nicotine and alcohol disorders (16). In a qualitative study with affected people, a high degree of stigmatization and a resulting tabooing of the disease were found (5). Impairments lead to feelings of helplessness and reduced independence. Also, self-esteem often decreases, due to self-loathing and shame brought on by, among others, the unpleasant odour that develops when abscesses rupture. HS restricts patients’ social as well as sexual life (5, 17). It can lead to a high burden of disease (18, 19) and is among the skin diseases that causes the greatest impairment of quality of life (QoL) (20).
Several generic dermatological health-related quality of life (HRQoL) measures have been used in HS (21), but these cannot reflect the specific impairments of patients with HS. The most widely used measure is the Dermatology Life Quality Index (DLQI) (22). The published HS-specific HRQoL instruments are lacking in full psychometric evaluation (23–29) as they showed limited evidence of validity and/or reliability or prolonged recall period (20). There is also a large gap in their development and use. Thus, there is a need for developing and implementing HS-specific HRQoL measures (30, 31). The HIdradenitis SuppuraTiva cORe outcomes set International Collaboration recommends that development should include physical functioning, psychological functioning, psychosocial functioning, emotional well-being, and ability to work or study (31). Furthermore, members of the European Association of Dermatology and Venereology Task Force on Quality of Life stated several advantages of measuring QoL in dermatological clinical practice (32), which were not directly addressed by any of the existing HRQoL measures to date.
This study aimed to develop and validate a new disease-specific patient-reported outcome measure (PROM) for the assessment of QoL in patients with HS for different study and clinical settings.
MATERIALS AND METHODS
The quality of life in hidradenitis suppurativa (QoL-HS) questionnaire was developed and validated based on the German guidance on assessing PROMs in dermatology (33) and international standards (34, 35).
Qualitative approaches to develop the quality of life in hidradenitis suppurativa questionnaire
For the initial item generation phase, 72 patients provided written information on their impairments due to HS in an open survey (semi-structured) (according to Blome et al. (36)). Patients were recruited in 5 German and 1 Swiss specialized care units. The data were subjected to content analysis (adapted from Mayring (37)). Thereby, the impairments described by the patients were categorized in order to build items reflecting these impairments. Then, the items were condensed in an expert panel consisting of 2 specialized dermatologists, 2 psychologists, and 2 patients. Finally, cognitive interviews were conducted iteratively to assess and improve the instrument, using think-aloud, probing, and paraphrasing techniques with patients differing in age, sex, and educational status, until no further adaptations to the questionnaire were necessary.
Quantitative validation of the quality of life in hidradenitis suppurativa questionnaire
The QoL-HS was validated in a non-interventional longitudinal study, reflecting clinical routine care. Dermatologists recruited patients in 5 specialized care units in Germany (consecutive sampling). Patients were eligible to participate if diagnosed with HS by a dermatologist and after providing written informed consent. The questionnaire development procedure was approved by the ethics committee of the Medical Association of Hamburg (PV4888).
To validate the QoL-HS, the following instruments were used:
- EuroQoL 5 Dimensions (EQ-5D) for measuring generic health-related quality of life (HRQoL) including the EuroQoL 5 Dimensions-3 levels (EQ5D-3L) and a visual analogue scale ranging from 0 to 100 (EuroQoL visual analogue (EQ VAS): 0=worst health; 100=best health) (38)
- Dermatology Life Quality Index (DLQI) (22) for dermatology-related QoL; the total sum score ranges from 0 to 30 with higher scores indicating greater impairment of QoL
- Hurley’s classification for measuring the need of surgical intervention of a non-inflammatory HS phenotype. The classification system allows the disease to be categorized into 1 of 3 severity levels (3)
- Hidradenitis Suppurativa Physician’s Global Assessment score (HS-PGA), which classifies disease severity into 6 different stages (0=clear; 5=very severe) (3).
Further clinical and demographic data were obtained, including affected body area, number of lesions, comorbidity, current therapies, age, sex, educational status, and questions on usability of, and satisfaction with, the QoL-HS were asked. Patients completed the questionnaires at their first visit (t1) and at a follow-up visit after 4–12 weeks’ treatment (t2). Dermatologists answered the questions on the patients’ clinical characteristics at the same 2 time-points.
Statistical analysis
Data analysis was conducted using SPSS for Windows version 26.0 (IBM, Armonk, NY, USA). Descriptive statistics were performed for the analysis of demography, usability/satisfaction, and all other parameters including item distribution of the QoL-HS. In order to determine validity of the QoL-HS, the analyses listed below was conducted:
- to test for structural validity of the QoL-HS, an explorative principal axes factor analysis with oblique rotation was performed on all items at t1. Factors with an eigenvalue of 1 or higher were extracted. All items were then assigned to the factor they loaded highest on, thereby grouping the items into subscales.
- to determine internal consistency within the subscales, Cronbach’s alphas were computed for the items of each subscale at t1.
- to ensure content validity, the target population was involved into both item generation and pilot testing through cognitive debriefing. In addition, the number of missing values per item at t1 was determined as an indicator of items irrelevant to the target population.
- to assess construct validity, the QoL-HS was correlated with other patient-reported (EQ-5D, DLQI) and clinical outcomes (HS-PGA, Hurley stage, number of inflammatory lesions) at t1 using Pearson correlation coefficient. A high correlation was assumed if the coefficient value was between ±0.50 and ±1, a medium correlation if it was between ± 0.30 and ±0.49, and a low correlation if it was below ±0.29 (39).
- To determine responsiveness (sensitivity to change) the values of QoL-HS and HRQoL measures were correlated (EQ5D-3L, DLQI) at t2, controlling for t1 HRQoL values performing partial correlations.
Translation
In a final step, the QoL-HS was independently translated into English by 2 professional translators who are English native speakers. The resulting English versions were each independently back-translated into German by 2 professional translators who were German native speakers. In a conference with the translators and questionnaire developers, we discussed each item until consensus on the final translation was reached (according to Eremenco et al. (40)).
RESULTS
Qualitative approaches and the quality of life in hidradenitis suppurativa questionnaire
Seventy-two patients participated in the open survey. Their mean ± standard deviation (SD) age was 40.4 ± 11.4 years and 58.3% were male. The survey resulted in 35 categorical themes regarding impairments. The expert panel discussed these categories and reduced the number of items to 23. Seven cognitive interviews with patients with HS were necessary to reach a questionnaire that required no further adaptations.
The resulting QoL-HS questionnaire consists of 23 items that assess QoL in the past week. Answers were given on a 5-point Likert scale (0=”not at all”; 4=”very”). The QoL-HS global score was computed by averaging all items. In case more than 25% of the items were missing, the score was not computed for the respective patient.
Sampling of the quantitative validation study
A total of 101 patients completed the questionnaires, mean age 38 years (median ± SD 48 ± 12; range: 19–75 years). Of these, 69 were female (68.3%). First symptoms of HS appeared 13 years before study inclusion on average (median 11; SD 10; range: 1–40 years), first diagnosis was made 7 years after onset of signs on average (median 4; SD 9; range: 0–39 years). At the time of survey, approximately one-third of patients were treated with long-term antibiotic therapy (n = 37; 36.6%), with biologics (n = 36; 35.6%) and with topical treatment (n = 30; 29.7%). At t1 mean DLQI was 11.5 (median ± SD 12 ± 6.8; range: 0–26), mean EQ-5D was 71.7 (median ± SD 75 ± 22.4; range: 20.5–100), and mean HS-PGA was 2.7 (median ± SD 3 ± 1.1; range: 0–5). 22 patients had Hurley stage 1 (21.8%), 56 Hurley stage II (55.4%), and 23 Hurley stage III (22.8%).
Structural validity
In the explorative factor analysis (t1), 75 patients without missing values in the HRQoL were included. This study found 4 factors with an eigenvalue greater than 1, which together explained 69.17 of overall variance. In a subsequent factor analysis, the number of factors was restricted to 2 because: (i) the scree plot suggested only 2 substantial factors with a bend visible between factor 2 and 3 and (ii) after rotation, only 2 of the factors remained with an eigenvalue above 1 explaining 59.21% of overall variance. The 2 subscales were interpreted (Table I) as follows:
| Item | Impairments (over the past 7 days) | Missings n | Mean (SD) (scale 0–4) | Mean (scale 0–10)* | ”Quite”/”very” % | Factor loadings | |
| Subscale 1** | Subscale 2** | ||||||
| 1 | I have experienced pain due to HS | 0 | 2.43 (1.27) | 6.08 | 56.5 | 0.50 | 0.64 |
| 2 | I have experienced itching due to HS | 1 | 2.02 (1.26) | 5.05 | 35.7 | 0.13 | 0.79 |
| 3 | I have experienced an unpleasant smell due to HS | 1 | 1.65 (1.29) | 4.13 | 26.7 | 0.11 | 0.80 |
| 4 | I have experienced oozing from the skin | 2 | 1.84 (1.37) | 4.60 | 34.7 | 0.04 | 0.80 |
| 5 | I have experienced inflamed skin | 2 | 2.51 (1.26) | 6.28 | 55.4 | 0.35 | 0.75 |
| 6 | My sleep has been affected by HS | 1 | 1.38 (1.29) | 3.45 | 23.7 | 0.36 | 0.70 |
| 7 | I have been unable to sit comfortably due to HS | 0 | 1.76 (1.56) | 4.40 | 37.6 | 0.61 | 0.38 |
| 8 | I have been unable to move around easily due to HS | 1 | 1.81 (1.36) | 4.53 | 31.6 | 0.70 | 0.50 |
| 9 | My enjoyment of life has been limited/impaired | 2 | 2.00 (1.38) | 5.00 | 41.5 | 0.73 | 0.43 |
| 10 | I have experienced shame due to HS | 3 | 2.08 (1.52) | 5.20 | 46.6 | 0.64 | 0.45 |
| 11 | I have experienced fear due to HS | 2 | 1.67 (1.44) | 4,18 | 30.7 | 0.72 | 0.18 |
| 12 | I have experienced financial worries due to HS | 1 | 0.76 (1.29) | 1.90 | 14.8 | 0.72 | –0.06 |
| 13 | I have felt helpless due to HS | 0 | 1.73 (1.39) | 4.33 | 36.7 | 0.77 | 0.29 |
| 14 | My day-to-day life has been impaired due to HS | 0 | 1.74 (1.28) | 4.35 | 34.6 | 0.74 | 0.46 |
| 15 | I have been unable to spend time with others due to HS | 0 | 0.93 (1.18) | 2.33 | 25.8 | 0.70 | 0.31 |
| 16 | My relationship has been strained due to HS | 1 | 1.10 (1.91) | 5.15 | 20.8 | 0.73 | 0.17 |
| 17 | My sexual life has been negatively affected due to HS | 10 | 1.78 (1.54) | 4.45 | 35.6 | 0.76 | 0.24 |
| 18 | My leisure time and sport activities have been negatively affected by HS | 2 | 1.95 (1.45) | 4.88 | 39.6 | 0.74 | 0.40 |
| 19 | The daily treatment of HS has been time consuming | 3 | 1.50 (1.27) | 3.75 | 27.8 | 0.64 | 0.42 |
| 20 | I have been encumbered with personal costs for the treatment of HS | 1 | 1.34 (1.30) | 3.35 | 21,8 | 0.63 | 0.11 |
| 21 | I have had to rely on the help of others due to HS | 2 | 1.06 (1.19) | 2.65 | 14.9 | 0.76 | 0.05 |
| 22 | I have been unable to take care of myself normally because of HS | 1 | 1.01 (1.21) | 2.53 | 15.9 | 0.66 | 0.19 |
| 23 | I have experienced side effects due to HS treatment | 6 | 0.82 (1.25) | 2.05 | 13.8 | 0.29 | 0.51 |
| *Scale 0–4 projected to a scale 0–10. **Subscale 1=social and psychological impairments; subscale 2=physical impairments; factor loadings in bold indicate that the item was assigned the respective subscale. SD: standard deviation. |
|||||||
- subscale 1 with 16 items reflecting social and psychological impairments;
- subscale 2 with 6 items reflecting physical impairments.
One item did not load on either of the 2 factors: “I have experienced side-effects due to hidradenitis suppurativa treatment”.
Distribution of quality of life in hidradenitis suppurativa scores
The mean QoL-HS total score at t1 was 1.60 (median ± SD 1.73 ± 0.48). The mean value of items ranged from 0.76 (least affected: “I have experienced financial worries due to hidradenitis suppurativa”) to 2.51 (most affected: “I have experienced inflamed skin”) (Table I). This study additionally projected all mean values of the 0–4 scale to a 0–10 scale for future comparisons with other scales (Table I). One patient had the highest observed global score of 3.13 (scale 0–4) and 2 patients the lowest possible score of 0 (2.0%). This means that no ceiling effect was detected, but a floor effect was detected. Most of the patients had a total score between 0.50 and 1.49 (Fig. 1). The highest scores were less often achieved.
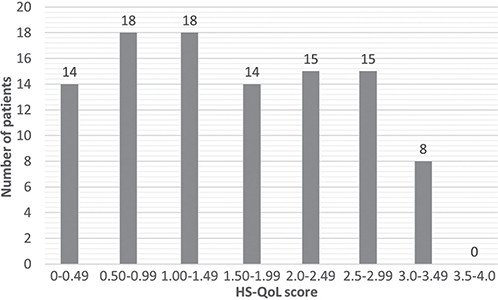
Fig. 1. Distribution of quality of life in hidradenitis suppurativa (QoL-HS) total score for N=101 patients.
The mean score of subscale 1 (social and psychological impairments) was 1.57 (median 1.74; SD 0.43; range: 0.76–2.08) and of subscale 2 (physical impairments) 1.97 (median 1.93; SD 0.44; range: 1.38–2.51). There were 6 items with a mean score above 2 (n = 3 in subscale 1; n = 3 in subscale 2). The 2 leading items were “I have experienced inflamed skin” and “I have experienced pain due to hidradenitis suppurativa” also having the highest number of patients choosing “quite”/”very” (Table I). Three items had a score below 1, indicating low impairment. Two of them can be found in subscale 1: “I have experienced financial worries due to hidradenitis suppurativa” (least impaired); “I have been unable to spend time with others due to hidradenitis suppurativa” (third least impaired). The second least impaired item is the one not assigned to a subscale: “I have experienced side-effects due to hidradenitis suppurativa treatment”.
Internal consistency
Cronbach’s alpha for the items of subscale 1 was 0.95 (n = 75). Corrected item-scale correlations ranged from 0.58 (“I have been encumbered with personal costs for the treatment of hidradenitis suppurativa”) to 0.83 (“My day-to-day life has been impaired due to hidradenitis suppurativa”). The Cronbach’s alpha for subscale 2 was 0.88 (n = 75) with corrected item-scale correlations ranging from 0.66 (“I have experienced oozing from the skin”) to 0.76 (“I have experienced inflamed skin”).
Missing values as an indicator of content validity
All QoL-HS total scores could be calculated as none of them had more than 25% missing values at t1. Two items had missing values higher than 5%: “My sexual life has been negatively affected due to hidradenitis suppurativa” (n = 10) and “I have experienced side-effects due to hidradenitis suppurativa treatment” (n = 6). Otherwise, items only showed missing values in between n = 0 and n = 3 (Table I).
Construct validity
The QoL-HS global score correlated significantly with PROMs (HRQoL measures) and with 2 of the 3 clinical outcomes (t1; Table II): medium-sized negative correlations between QoL-HS and the generic HRQoL outcomes were found (EQ5D-3L: r = –0.49; EQ5D-VAS: r = –0.46) and a high positive correlation between QoL-HS and the dermatology-specific QoL instrument (DLQI: r = 0.69). Lower correlations were found between QoL-HS and HS-PGA (r = 0.21) as well as the number of lesions (r = 0.22). There was no significant correlation between QoL-HS and Hurley stage (r = 0.07). For the subscales, correlations were similar. In addition, subscale 1 (social and psychological impairments) correlated more highly with the PROMs than subscale 2 (physical impairments), and subscale 2 correlated more highly with the clinical outcome measures than subscale 1.
Responsiveness
Controlling for t1 values of HRQoL measures, significant and high correlations were found: the correlation with the generic HRQoL (EQ5D-3L at t2) was negative and with the dermatology-specific HRQoL (DLQI at t2) was positive (Table II). Regarding the subscales, the correlations were also similar, except for the correlations of subscale 2, which were medium instead of large.
Usability and satisfaction
A great majority of patients evaluated the QoL-HS questionnaire positively (Table III). They indicated that they were sufficiently informed about the purpose of the QoL-HS (94.1%), that instructions (94.1%) and questions (95.0%) were very understandable, and the questionnaire was easy to read (96%). In their opinion, all important questions were asked (96.0%) and they had no difficulty choosing an answer (87.0%).
| Items* | Yes n (%) | No n (%) | Missings n (%) |
| Do you feel sufficiently informed about the purpose of the questionnaire? | 95 (94.1) | 4 (4.0) | 2 (2.0) |
| Did you find the instructions on how to fill in the questionnaire understandably worded? | 95 (94.1) | 3 (3.0) | 3 (3.0) |
| Did you find the questions understandably formulated? | 96 (95.0) | 3 (3.0) | 2 (2.0) |
| Was the questionnaire easy to read for you? | 97 (96.0) | 2 (2.0) | 2 (2.0) |
| Do you feel that questions important to you are missing? | 2 (2.0) | 97 (96.0) | 2 (2.0) |
| Were there any questions where you had a hard time deciding on an answer? | 11 (10.9) | 88 (87.1) | 2 (2.0) |
| *Items relate to the QoL-HS questionnaire and to another simultaneously developed questionnaire on patients’ goals and benefits including the same items as the QoL-HS. | |||
DISCUSSION
HS can lead to a high burden of disease and impairment of QoL. There is a lack of validation and little experience of the use of HRQoL measures for studies and clinical practice. This study aimed to develop and validate a new disease-specific PROM for the assessment of QoL in patients with HS usable for both settings: the QoL-HS.
The QoL-HS can be divided in 2 subscales, allowing for more detailed interpretation of data. There were higher correlations between subscale 1 (social and psychological impairments) and PROMs and between subscale 2 (physical impairments) and clinical outcome measures. This, again, shows that the 2 subscales make sense in terms of content and that it is especially important to also consider social and psychological impairments within studies and clinical practice.
The QoL-HS global score and subscale scores also correlated significantly with other patient-reported HRQoL measures, but plausibly especially high with the dermatology-specific measure. The latter also assesses the patients’ impairments retrospectively, as the QoL-HS does, while the other measures ask for the dailies or current state in general. Significant correlations were also found between QoL-HS and clinical outcomes, but they were lower than between QoL-HS and PROMs. An explanation might be that all PROMs (including QoL-HS) reflect the patients’ perspective and it differs from the dermatologists’ perspective. The literature shows that HRQoL correlates only partly or even poorly with the severity of a disease (41), again showing the high importance of collecting PROM data in both clinical studies and clinical practice.
There was no significant correlation between QoL-HS and Hurley stage. This is understandable, since Hurley stage is a static measurement instrument. Dynamic changes, especially in inflammatory activity, are not reflected by this measurement instrument.
This study also found significant and high correlations of change in QoL-HS with change in other PROMs, indicating responsiveness.
Existing tools differ from the QoL-HS regarding different criteria (20). The number of items is between 10 and 44, some using 5-point Likert scales and some visual analogue scales (VAS). The HiSQoL and the HS-QoL-24 are the most widely used HRQoLs for HS, as they have a usable number of items (17 and 24) and use 5-point Likert scales. In comparison with the QoL-HS, the recall period of the HSQoL-24 is 4 weeks (28) and the HiSQoL was explicitly developed and tested for clinical trials. That is why it only includes items with “concepts related to active disease”, while “concepts related to secondary skin damage” (e.g. scarring) were excluded (42). Most of the existing tools only conducted qualitative development studies (29), pilot testing (23, 26), or only included 30 (28) to 40 (25) patients within the validation procedure. The development and validation of the QoL-HS included both qualitative and quantitative approaches with a statistically appropriate sample size, resulting in a valid instrument with no restrictions related to content or scope of application. On the QoL-HS, patients use 23 items on a 5-point Likert scale to indicate how much HS has affected their lives in the past week. The current data show a high usability and acceptance of the QoL-HS, which is indispensable for a successful implementation. The subscales of the existing HRQoL measures also differ from the subscales of the QoL-HS: some are unidimensional, others consist of 3–7 subscales, e.g. symptoms, sexual activity consequences, work consequences, activity adaptation, psychosocial, economic (20). The QoL-HS is divided in 2 subscales only. On the one hand, this allows for less information; on the other hand, the questionnaire consists of 23 items only, which can be divided into 2 reasonable and clear subscales, which enable researchers and clinicians to obtain a clear summary of the data. This again leads to high usability.
These results indicate that the QoL-HS is an internally consistent, valid, responsive, and usable instrument to assess QoL in patients with HS. It is suitable for studies on QoL including clinical studies and for routine care. To facilitate future usage of the QoL-HS, the 0–4 scale was projected to a 0–10 scale for easier comparisons with other scores. We plan to digitalize the instrument and translate it into different languages, including further validation studies. This instrument may support the understanding of patients’ impairments and enable improvements in patient care.
ACKNOWLEDGEMENTS
The authors thank the Scientific Communication Team of the IVDP, in particular Merle Twesten and Mario Gehoff, for copyediting. The Departments of Dermatology, Venereology, Allergology and Immunology, Dessau Medical Center, Dessau, Germany are healthcare providers of the European Reference Network for Rare and Complex Skin Diseases (ERN Skin-ALLOCATE Skin group).
REFERENCES
- Saunte DML, Jemec GBE. Hidradenitis suppurativa: advances in diagnosis and treatment. JAMA 2017; 318: 2019–2032.
- Goldburg SR, Strober BE, Payette MJ. Hidradenitis suppurativa: epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol 2020; 82: 1045–1058.
- Napolitano M, Megna M, Timoshchuk EA, Patruno C, Balato N, Fabbrocini G, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol 2017; 10: 105–115.
- Zouboulis CC, Del Marmol V, Mrowietz U, Prens EP, Tzellos T, Jemec GBE. Hidradenitis suppurativa/acne inversa: criteria for diagnosis, severity assessment, classification and disease evaluation. Dermatology 2015; 231: 184–190.
- Esmann S, Jemec GBE. Psychosocial impact of hidradenitis suppurativa: a qualitative study. Acta Derm Venereol 2011; 91: 328–332.
- Jfri A, Nassim D, O’Brien E, Gulliver W, Nikolakis G, Zouboulis CC. Prevalence of hidradenitis suppurativa: a systematic review and meta-regression analysis. JAMA Dermatol 2021; 157: 924–931.
- Cosmatos I, Matcho A, Weinstein R, Montgomery MO, Stang P. Analysis of patient claims data to determine the prevalence of hidradenitis suppurativa in the United States. J Am Acad Dermatol 2013; 69: 819.
- Theut Riis P, Pedersen OB, Sigsgaard V, Erikstrup C, Paarup HM, Nielsen KR, et al. Prevalence of patients with self-reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross-sectional study. Br J Dermatol 2019; 180: 774–781.
- Kirsten N, Petersen J, Hagenström K, Augustin M. Epidemiology of hidradenitis suppurativa in Germany – an observational cohort study based on a multisource approach. J Eur Acad Dermatol Venereol 2020; 34: 174–179.
- Kirsten N, Zander N, Augustin M. Prevalence and cutaneous comorbidities of hidradenitis suppurativa in the German working population. Arch Dermatol Res 2021; 313: 95–99.
- Jemec GB, Heidenheim M, Nielsen NH. The prevalence of hidradenitis suppurativa and its potential precursor lesions. J Am Acad Dermatol 1996; 35: 191–194.
- Vekic DA, Frew JW, Woods J, Cains GD. Adopting the orphan: the importance of recognising hidradenitis suppurativa as a systemic auto-inflammatory disease. Australas J Dermatol 2016; 57: 69–70.
- Wolk K, Join-Lambert O, Sabat R. Aetiology and pathogenesis of hidradenitis suppurativa. Br J Dermatol 2020; 183: 999–1010.
- Nikolakis G, Kokolakis G, Kaleta K, Wolk K, Hunger R, Sabat R, et al. Pathogenese der hidradenitis suppurativa/acne inversa. Hautarzt 2021; 72: 658–665.
- Shavit E, Dreiher J, Freud T, Halevy S, Vinker S, Cohen AD. Psychiatric comorbidities in 3207 patients with hidradenitis suppurativa. J Eur Acad Dermatol Venereol 2015; 29: 371–376.
- Mavrogiorgou P, Juckel G, Reimelt A, Hessam S, Scholl L, Frajkur JL, et al. Psychiatrische Komorbidität bei Hidradenitis suppurativa/Acne inversa. Hautarzt 2021; 72: 426–434.
- Balieva F, Kupfer J, Lien L, Gieler U, Finlay AY, Tomás-Aragonés L, et al. The burden of common skin diseases assessed with the EQ5D™: a European multicentre study in 13 countries. Br J Dermatol 2017; 176: 1170–1178.
- Wolkenstein P, Loundou A, Barrau K, Auquier P, Revuz J. Quality of life impairment in hidradenitis suppurativa: a study of 61 cases. J Am Acad Dermatol 2007; 56: 621–623.
- Matusiak L, Bieniek A, Szepietowski JC. Psychophysical aspects of hidradenitis suppurativa. Acta Derm Venereol 2010; 90: 264–268.
- Chernyshov PV, Finlay AY, Tomas-Aragones L, Poot F, Sampogna F, Marron SE, et al. Quality of life in hidradenitis suppurativa: an update. Int J Environ Res Public Health 2021; 18.
- Zouboulis CC, Chernyshov PV. Hidradenitis suppurativa-specific, patient-reported outcome measures. J Eur Acad Dermatol Venereol 2021; 35: 1420–1421.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI) – a simple practical measure for routine clinical use. Clin Exp Dermatol 1994; 19: 210–216.
- Pinard J, Vleugels RA, Joyce C, Merola JF, Patel M. Hidradenitis suppurativa burden of disease tool: Pilot testing of a disease-specific quality of life questionnaire. J Am Acad Dermatol 2018; 78: 215-217.e2.
- Thorlacius L, Esmann S, Miller I, Vinding G, Jemec GBE. Development of HiSQOL: a hidradenitis suppurativa-specific quality of life instrument. Skin Appendage Disord 2019; 5: 221–229.
- Kimball AB, Sundaram M, Banderas B, Foley C, Shields AL. Development and initial psychometric evaluation of patient-reported outcome questionnaires to evaluate the symptoms and impact of hidradenitis suppurativa. J Dermatolog Treat 2018; 29: 152–164.
- Sisic M, Kirby JS, Boyal S, Plant L, McLellan C, Tan J. Development of a quality-of-life measure for hidradenitis suppurativa. J Cutan Med Surg 2017; 21: 152–155.
- McLellan C, Sisic M, Oon HH, Tan J. Preliminary validation of the HS-QoL: a quality-of-life measure for hidradenitis suppurativa. J Cutan Med Surg 2018; 22: 142–146.
- Marrón SE, Gómez-Barrera M, Tomás-Aragonés L, Díaz Díaz RM, Vilarrasa Rull E, Madrid Álvarez MB, et al. Desarrollo y validación preliminar del instrumento HSQoL-24 para evaluar calidad de vida en pacientes con hidradenitis supurativa. Actas Dermosifiliogr (Engl Ed) 2019; 110: 554–560.
- Chiricozzi A, Bettoli V, Pità O de, Dini V, Fabbrocini G, Monfrecola G, et al. HIDRAdisk: an innovative visual tool to assess the burden of hidradenitis suppurativa. J Eur Acad Dermatol Venereol 2019; 33: e24-e26.
- Chernyshov PV, Zouboulis CC, Tomas-Aragones L, Jemec GB, Svensson A, Manolache L, et al. Quality of life measurement in hidradenitis suppurativa: position statement of the European Academy of Dermatology and Venereology task forces on Quality of Life and Patient-Oriented Outcomes and Acne, Rosacea and Hidradenitis Suppurativa. J Eur Acad Dermatol Venereol 2019; 33: 1633–1643.
- Thorlacius L, Ingram JR, Villumsen B, Esmann S, Kirby JS, Gottlieb AB, et al. A core domain set for hidradenitis suppurativa trial outcomes: an international Delphi process. Br J Dermatol 2018; 179: 642–650.
- Finlay AY, Salek MS, Abeni D, Tomás-Aragonés L, van Cranenburgh OD, Evers AWM, et al. Why quality of life measurement is important in dermatology clinical practice: an expert-based opinion statement by the EADV Task Force on Quality of Life. J Eur Acad Dermatol Venereol 2017; 31: 424–431.
- Augustin M, Amon U, Braathen L, Bullinger M, Gieler U, Klein GF, et al. Erfassung von Lebensqualität in der Dermatologie. J Dtsch Dermatol Ges 2004; 2: 802–806.
- Aaronson N, Alonso J, Burnam A, Lohr KN, Patrick DL, Perrin E, et al. Assessing health status and quality-of-life instruments: attributes and review criteria. Qual Life Res 2002; 11: 193–205.
- Mokkink LB, Prinsen CAC, Patrick DL, Alonso J, Bouter LM, Vet HCW de, et al. COSMIN study design checklist for patient-reported outcome measurement instruments; 2019.
- Blome C, Augustin M, Heyer K, Knöfel J, Cornelsen H, Purwins S, et al. Evaluation of patient-relevant outcomes of lymphedema and lipoedema treatment: development and validation of a new benefit tool. Eur J Vasc Endovasc Surg 2014; 47: 100–107.
- Mayring P. Qualitative content analysis: a step-by-step guide / Philipp Mayring. 1st. Los Angeles: SAGE; 2021.
- EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990; 16: 199–208.
- Freedman D, Pisani R, Purves R. Statistics. International student edn, 4th edn / David Freedman, Robert Pisani, Roger Purves. New York: W.W. Norton & Co.; 2007.
- Eremenco S, Pease S, Mann S, Berry P. Patient-Reported Outcome (PRO) Consortium translation process: consensus development of updated best practices. J Patient Rep Outcomes 2017; 2: 12.
- Schäfer I, Hacker J, Rustenbach SJ, Radtke M, Franzke N, Augustin M. Concordance of the Psoriasis Area and Severity Index (PASI) and patient-reported outcomes in psoriasis treatment. Eur J Dermatol 2010; 20: 62–67.
- Kirby JS, Thorlacius L, Villumsen B, Ingram JR, Garg A, Christensen KB, et al. The Hidradenitis Suppurativa Quality of Life (HiSQOL) score: development and validation of a measure for clinical trials. Br J Dermatol 2020; 183: 340–348.