Basal cell carcinoma (BCC) is the most common malignant cutaneous neoplasm that predominantly affects the face and neck. Diagnosis of BCC has been greatly improved by dermoscopy, which is associated with high sensitivity and specificity (1, 2). However, when tumours are small in size, classic dermoscopic features, such as large blue-grey ovoid nests, multiple blue-grey globules, and arborizing vessels, are frequently unclear or are only partially present, which makes an accurate diagnosis difficult (4–8). Capillaroscopy (e.g. nailfold video-capillaroscopy, nailfold capillaroscopy, and video-capillaroscopy) has been used to evaluate peripheral circulatory disorders and vascular abnormalities in collagen diseases, such as systemic sclerosis (9). While dermoscopy has a magnification of approximately ×10 to ×30, capillaroscopy (GOKO Bscan-Z; GOKO Imaging Devices Co., Ltd, Kanagawa, Japan, https://www.gokocamera.com/english/ev/bscan-z.php) has a remarkable magnification capability in the range of ×145 to ×590, and enables us to observe the morphology of capillaries and measure the blood flow velocity and vessel diameter with high resolution. Moreover, capillaroscopy is a non-invasive examination that can be performed by simply touching the surface. To investigate the usefulness of capillaroscopy for the diagnosis of BCC, 4 lesions less than 3-mm diameter were examined.
METHODS and CASE REPORTS
Polarized dermoscopy images were obtained using a DZ-D100 (Casio Computer Co., Ltd, Tokyo, Japan, https://dz-image.casio.jp/products/derm/dz_d100.html). Capillaroscopy images were obtained using a GOKO Bscan-Z (GOKO Imaging Devices Co., Ltd). Ethical approval for this study was granted by the ethics commission of Gunma University. All patients provided written informed consent.
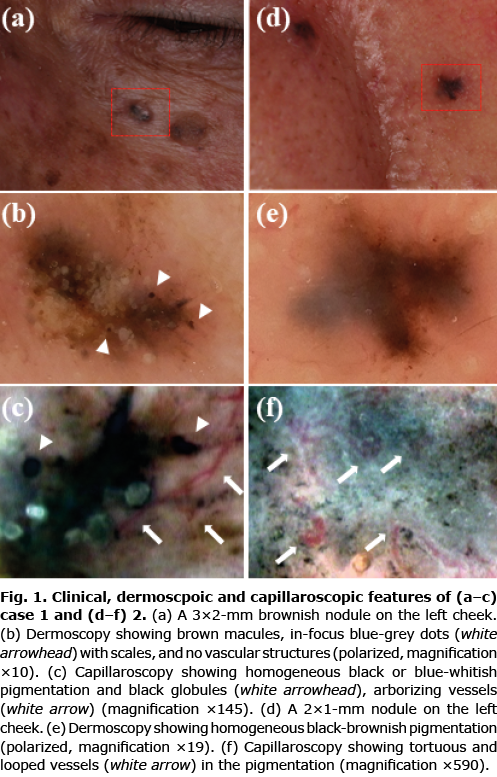
Case 1. A 72-year-old Japanese man presented a small brownish nodule on the left cheek. Physical findings showed a 3×2-mm papule on the left cheek (Fig. 1a). Dermoscopic examinations showed relatively uniform brown macules with no vascular structures and a few in-focus blue-grey dots (Fig. 1b). Capillaroscopy examinations revealed homogeneous black or blue-whitish pigmentation and blue-grey dots and arborizing vessels (Fig. 1c, Fig. S1a, b). Wide excision was performed with a 2-mm horizontal margin. A histopathological examination revealed nodular BCC (Fig. S1c).
Case 2. A 76-year-old Japanese man presented with 2×1 mm black nodule on his left cheek (Fig. 1d). Dermoscopic examinations showed homogeneous black-brownish pigmentation (Fig. 1e). Capillaroscopy examinations revealed homogeneous blue-black pigmentation (Fig. 1f). On high magnification, tortuous and looped vessels were observed (Fig. S1d, e). No similar changes were seen in the surrounding area. Surgical excision was performed with a 1-mm horizontal margin. Histopathological findings revealed hyperpigmented nodular BCC (Fig. S1f).
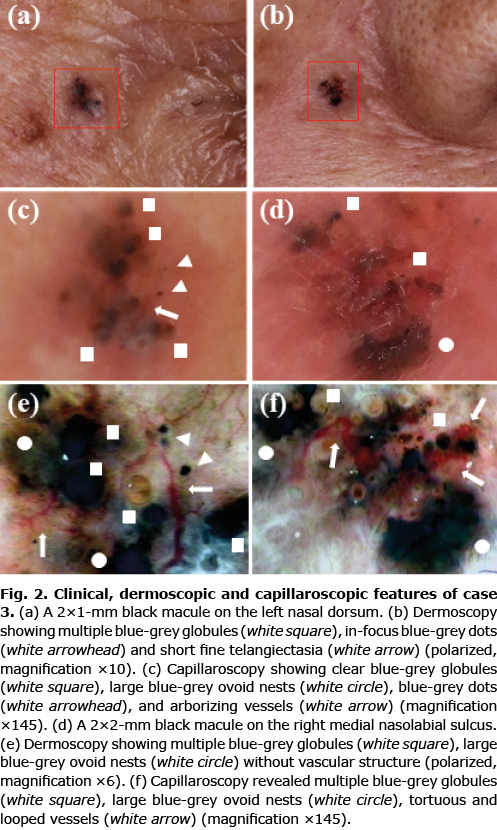
Case 3. An 80-year-old Japanese woman presented with multiple small black macules. Physical examinations showed a 2×1-mm black macule on the left nasal dorsum (Fig. 2a) and a 2×2-mm black macule on the right medial nasolabial sulcus (Fig. 2b). Dermoscopic examinations showed small multiple blue-grey globules, in-focus blue-grey dots, short fine telangiectasia (SFT) (Fig. 2c) and in-focus blue-grey dots, large blue-grey ovoid nests (Fig. 2d), respectively. Capillaroscopic examinations revealed clear blue-grey globules, large blue-grey ovoid nests, blue-grey dots, and arborizing vessels (Fig. 2e, f, Fig. S2a) and multiple blue-grey globules, large blue-grey ovoid nests, tortuous and irregular looped vessels (Fig. S2b–d). Both pigmented macules were excised with a 2-mm horizontal margin. A histopathological examination revealed micronodular and nodular BCC, respectively (Fig. S2e,f).
DISCUSSION
This is the first case series suggesting the usefulness of capillaroscopy in small pigmented BCC. The classic or non-classic dermoscopic features of BCC include arborizing vessels and SFT (3, 10). In all cases, capillaroscopy was able to detect vascular structures that were not clarified or identified by dermoscopy. Arborizing vessels are defined as telangiectasias with tree-like branching, while SFT is defined as short (≤ 1 mm) vessels without tree-like branching (10, 11). In case 3, SFT was clearly observed as tree-like branching of vessels.
Furthermore, not only vascular structures but also pigmented structures such as blue-gray globules and in-focus blue-gray dots, could be clearly observed by capillaroscopy. Capillaroscopy increases the detection rate of classic features and enables a more accurate diagnosis. Whether these findings were specific to BCC, we examined intradermal naevi case (Fig. S3a–c). Capillaroscopy showed multiple bule gray globules but no distinct vascular structures (Fig. S3d–f). This result suggests that capillaroscopy may be useful in differentiating small BCC.
Only 5 reports regarding the dermoscopic features of small BCC have been published (Table SI). Takahashi et al. (4) reported that arborizing vessels were absent, but multiple blue-gray globules and large blue-gray ovoid nests were frequently observed in small BCC (< 3 mm) (4). Pampena et al. (5) reviewed 12 BCCs (< 5 mm) and reported that arborizing vessels and large blue-ovoid nests were found in 6 and 5 cases. Sanchez-Martin et al. (6) compared 34 BCCs (≤3 mm) with 66 BCCs (3.1–5 mm) and reported no significant difference in the dermoscopic features. Popadic et al. (7) reported a higher frequency of arborizing vessels and SFT in larger BCC (> 1 cm). Longo et al. (8) revealed a significant difference in small BCCs (< 5 mm) for pigmented structures, but not for vascular structures. Among our cases, only case 3 had classic dermoscopic features. Because capillaroscopy allows for observation at a higher magnification than dermoscopy, it may be useful in smaller BCCs. In a study of BCCs (< 5 mm), arborizing vessels were observed in less than half of the cases (5, 6, 8). However, in smaller pigmented BCCs (< 3 mm), It may be difficult to confirm the specific vascular structure by dermoscopy. Furthermore, tortuous and irregular looped vessels were seen in Cases 2 and 3. SFT and hairpin vessels on dermoscopy can be visualized as tortuous and irregular looped vessels on capillaroscopy.
To date, there has only been one report on the use of capillaroscopy for BCC. Newell et al. examined BCC by capillaroscopy (magnification: ×77) and quantitatively compared the vascular structures of BCC to control skin or actinic keratosis (12). Although it was not used for diagnostic purposes, their findings were consistent with our study in that tortuous and looped vessels were increased in BCC. BCC frequently develops on the face and neck and may occur as multiple lesions (13). Therefore, the detection of smaller and earlier BCC by capillaroscopy can lead to less-invasive surgical resection. In addition, capillaroscopy is completely non-invasive and can be used easily and quickly at the bedside. Capillaroscopy can be a new diagnostic tool for smaller lesions of pigmented BCC.
REFERENCES
- Menzies SW, Westerhoff K, Rabinovitz H, Kopf AW, McCarthy WH, Katz B. Surface microscopy of pigmented basal cell carcinoma. Arch Dermatol 2000; 136: 1012–1016.
- Reiter O, Mimouni I, Dusza S, Halpern AC, Leshem YA, Marghoob AA. Dermoscopic features of basal cell carcinoma and its subtypes: a systematic review. J Am Acad Dermatol 2021; 85: 653–664.
- Marghoob AA, Braun R. Proposal for a revised 2-step algorithm for the classification of lesions of the skin using dermoscopy. Arch Dermatol 2010; 146: 426–428.
- Takahashi A, Hara H, Aikawa M, Ochiai T. Dermoscopic features of small size pigmented basal cell carcinomas. J Dermatol 2016; 43: 543–546.
- Pampena R, Specchio F, Ragazzi M, Pellacani G, Longo C. Dermoscopy and confocal microscopy of small sized basal cell carcinoma (diameter less than 5 mm). G Ital Dermatol Venereol 2020; 155: 116–118.
- Sanchez-Martin J, Vazquez-Lopez F, Perez-Oliva N, Argenziano G. Dermoscopy of small basal cell carcinoma: study of 100 lesions 5 mm or less in diameter. Dermatol Surg 2012; 38: 947–950.
- Popadic M, Vukicevic J. What is the impact of tumour size on dermoscopic diagnosis of BCC? J Eur Acad Dermatol Venereol 2015; 29: 2474–2478.
- Longo C, Specchio F, Ribero S, Coco V, Kyrgidis A, Moscarella E, et al. Dermoscopy of small-size basal cell carcinoma: a case-control study. J Eur Acad Dermatol Venereol 2017; 31: e273–e274.
- Pizzorni C, Cutolo M, Sulli A, Ruaro B, Trombetta AC, Ferrari G, et al. Long-term follow-up of nailfold videocapillaroscopic changes in dermatomyositis versus systemic sclerosis patients. Clin Rheumatol 2018; 37: 2723–2729.
- Altamura D, Menzies SW, Argenziano G, Zalaudek I, Soyer HP, Sera F, et al. Dermatoscopy of basal cell carcinoma: morphologic variability of global and local features and accuracy of diagnosis. J Am Acad Dermatol 2010; 62: 67–75.
- Giacomel J, Zalaudek I. Dermoscopy of superficial basal cell carcinoma. Dermatol Surg 2005; 31: 1710–1713.
- Newell B, Bedlow AJ, Cliff S, Drysdale SB, Stanton AW, Mortimer PS. Comparison of the microvasculature of basal cell carcinoma and actinic keratosis using intravital microscopy and immunohistochemistry. Br J Dermatol 2003; 149: 105–110.
- Kiiski V, de Vries E, Flohil SC, Bijl MJ, Hofman A, Stricker BH, et al. Risk factors for single and multiple basal cell carcinomas. Arch Dermatol 2010; 146: 848–855.