Patients with chronic itch describe their pruritus in a wide variety of ways. However, these subjective descriptions are often not taken into consideration by physicians. This study aimed to validate patients’ descriptions of pruritus, and to investigate the relationship between various descriptions of pruritus and the patient burden of chronic pruritus by examining the mediating effects of sleep disturbance and sexual dysfunction on patient’s quality of life, as predicted by various descriptions of pruritus. Exploratory and confirmatory factor analyses were performed to identify the factor structure measured by 11 descriptions of pruritus. The study then analysed differences in the degree of sleep disturbance, sexual dysfunction, and quality of life deterioration factors using a structural equation modelling method. Using data from 419 patients with chronic pruritus, 11 descriptions of pruritus were classified into 2 groups: (i) sensory pruritus (i.e. stinging, stabbing, burning, painful, formication, throbbing, and cold) that are linked with descriptions of pruritus patterns; and (ii) affective pruritus (i.e. annoying, unbearable, worrisome, and warm) from patient reports of psychological or emotional distress. The study found that affective pruritus decreases patient’s quality of life either directly or indirectly through sleep disturbance. In conclusion, clues about a patients’ sleep disturbance or poor quality of life can be obtained through their descriptions of pruritus.
Key words: chronic pruritus; description; sexual dysfunction; sleep disturbance; quality of life.
Accepted Nov 17, 2022; Published Nov 24, 2022
Acta Derm Venereol 2022; 102: adv00819.
DOI: 10.2340/actadv.v102.2527
Corr: Yong Hyun Jang, Department of Dermatology, School of Medicine, Kyungpook National University, 130, Dongduk-ro, Jung-gu, Daegu, Republic of Korea, and Seong-Jin Kim, Department of Dermatology, Chonnam National University Hospital, 42 Jebong-ro, Dong-gu, Gwangju, Republic of Korea. E-mail: yhjang@knu.ac.kr; seongkim@chonnam.ac.kr
SIGNIFICANCE
Chronic pruritus is expressed in very different ways by patients. Various expressions of chronic pruritus are often not noticed by dermatologists. In this study, 11 expressions of pruritus were classified into two groups: (i) sensory pruritus (i.e. sting, stabbing, burning, painful, formication, throbbing, and cold) (ii) affective pruritus (i.e. annoying, unbearable, worrisome, and warm). Of the two groups, affective pruritus had a more serious effect on the quality of life of the patient. This study provides clues to predict the degree of deterioration in quality of life through the patients’ pruritus expression.
INTRODUCTION
Pruritus, otherwise known as itch, is defined as an unpleasant sensation of the skin that provokes the urge to scratch (1). Given that it is a subjective symptom, clinicians rely primarily on patient descriptions to understand whether a patient is experiencing pruritus as a standalone symptom or as a manifestation of skin or systemic diseases. The sensation of pruritus is multidimensional with a complex transmission process; therefore, patients describe it in various ways, including stinging, stabbing, burning, painful, formication, throbbing, annoying, or unbearable (2–4). Some patients even describe pruritus with a sense of temperature (i.e. cold and hot sensations) (5–7). However, the specific expressions used by patients are often not taken into consideration, except in special cases such as neuropathic pruritus.
Several studies have identified descriptors of pruritus associated with specific skin disorders (3, 5, 7–9). Brenaut et al. (5) revealed that hot sensations, stinging, pinching, and stabbing were significantly more frequent in atopic dermatitis than in non-atopic eczema, urticaria, psoriasis and scabies. In addition, Lim et al. (9) reported that patients with pruritic acne describe pruritus using expressions such as tickling, crawling, stinging, and burning, or by using affective descriptors, such as annoying, bothersome, worrisome, and unbearable. However, these studies focused only on the association of pruritus descriptors with specific skin disorders and lacked interest in their association with itch-related quality of life (ItchyQoL).
Patients with pruritus experience impaired health-related quality of life and disrupted sleep, as well as fatigue due to scratching at night (10–13). A negative influence of pruritus on sleep quality is seen in chronic inflammatory skin disorders, such as atopic dermatitis and psoriasis. In addition, sexual dysfunction has been reported to be correlated with the presence of significant pruritus (14–16). Although there have been many reports on the effect of chronic pruritus, most studies have focused only on its intensity and not on descriptions of pruritus. Only a few studies have assessed the qualitative characteristics of pruritus in specific diseases. Yosipovitch et al. (2) analysed the relationship between pruritus quality and intensity in patients with uraemic pruritus, and Zachariae et al. (8) analysed the dimension of pruritus quality and its association with psychological symptoms and quality of life.
The aim of this study was to categorize and compare various descriptions of pruritus in patients with chronic pruritus according to their relationships with sleep quality, sexual dysfunction, and ItchyQoL.
MATERIALS AND METHODS
Patients
This nationwide questionnaire-based study was conducted in 11 hospitals in Korea from July 2016 to January 2017. A total of 419 patients (aged ≥ 20 years) who presented with chronic (> 6 weeks) pruritus, regardless of cause, were enrolled. The study protocol complied with the principles of the Declaration of Helsinki and was approved by the Institutional Review Board of Kyungpook National University Hospital, Daegu, Republic of Korea (IRB number KNUH 2021-01-036).
Pruritus descriptors
Descriptions of pruritus were constructed using a list of 11 words: stinging, stabbing, burning, annoying, unbearable, worrisome, painful, formication, throbbing, cold, and warm. The first 6 pruritus descriptors (stinging/stabbing/burning/annoying/unbearable/worrisome) included in the Itch Severity Scale (ISS), which is used as a multidimensional pruritus assessment tool, were chosen for this study (17). Although this study mainly utilized the ISS questionnaire, the descriptors within the ISS were somewhat limited. Thus, the descriptors were extended from the literature via field experts, including 15 dermatologists, and 11 descriptors were implemented that fit the pruritus severity and study population (2). Each descriptor was rated on a 4-point Likert scale ranging from 0 (none) to 3 (severe).
Sleep disturbance, sexual dysfunction, and quality of life survey
Sleep induction (difficulty falling asleep due to pruritus) and maintenance (awakening due to pruritus) were assessed as aspects of sleep disturbance. Information on sexual dysfunction was also obtained. Sleep disturbance and sexual dysfunction were evaluated using the ISS, which has been verified and used as a multidimensional scale for pruritus evaluation (17). The patients’ health-related quality of life was surveyed using a Korean translation of the pruritus-specific ItchyQoL questionnaire used in our previous study (18, 19).
Factor analysis on pruritus descriptors
To identify or confirm the structure of the underlying constructs (or factors) that are measured by items in the questionnaire, exploratory factor analysis (EFA) and confirmatory factor analysis (CFA) (20, 21) were conducted. In these factor analyses, EFA was applied with the obtained baseline data and CFA with the follow-up data after 3 h. This procedure allowed us to identify and confirm the factor structure of descriptions of pruritus. Furthermore, this study examined the reliability and validity of the factors identified using the baseline data, which are presented in the results section.
The assumptions of the factor analysis were checked by checking normality, multicollinearity, and outliers of pruritus descriptors. Based on the skewness and kurtosis of each description of pruritus, the study examined normality with a criterion of values between –3 and 3. All of the items (except kurtoses of “stabbing” and “cold” at both baseline and follow-up data) were satisfied with the criterion (Table SI). Multicollinearity was examined by evaluating the variance inflation factor (VIF). No VIF value was above 5, which confirmed that there was no multicollinearity. Using Cook’s distance, no univariate or multivariate outliers were observed.
Psychometrics: reliability and validity
Factor reliability. As a psychometric property of items indicating the degree to which factor scores are precise, the reliabilities of factors were examined using factor rho reliability (ρ ̂) (22, 23). Factor rho reliability is defined as the ratio of explained variance to total variance from the CFA parameters:
where  is the sum of the estimated unstandardized factor loadings among the indicators of the same factor,
is the sum of the estimated unstandardized factor loadings among the indicators of the same factor, 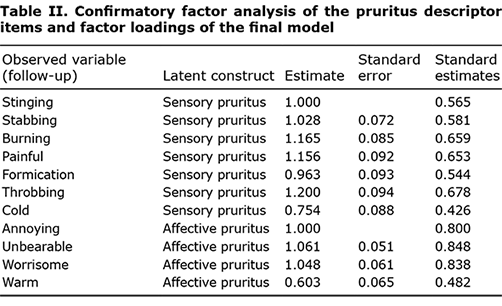 is the estimated factor variance, and
is the estimated factor variance, and 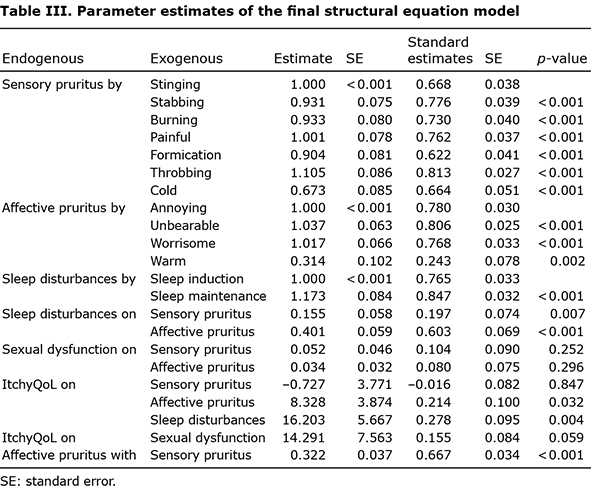 is the sum of the unstandardized error variances of those indicators. In CFA, factor loadings, error variances, and error covariances were estimated, which influence the true and total variance. Thus, to measure factor reliability within the CFA model, factor reliability facilitating CFA estimates is a preferred method for computing Cronbach’s alpha, assuming that all items hold the same distributions of mean and standard deviations, and their errors are uncorrelated (21).
is the sum of the unstandardized error variances of those indicators. In CFA, factor loadings, error variances, and error covariances were estimated, which influence the true and total variance. Thus, to measure factor reliability within the CFA model, factor reliability facilitating CFA estimates is a preferred method for computing Cronbach’s alpha, assuming that all items hold the same distributions of mean and standard deviations, and their errors are uncorrelated (21).
Construct validity. In the CFA, construct validity was examined with respect to convergent validity and discriminant validity, in addition to showing that factor loadings are greater than 0.45 (21). These indexes of convergent and discriminant validities provide support for how the underlying constructs are measured accurately and separately from each other, respectively. The CFA results provide evidence of how strongly indicators of a latent variable are interrelated (convergent validity) and how weakly latent variables are correlated (discriminant validity) (21). Convergent validity was provided by obtaining factor reliabilities greater than 0.70, and discriminant validity was provided by obtaining factor correlations lower than 0.80 (21, 24).
Structural equation modelling
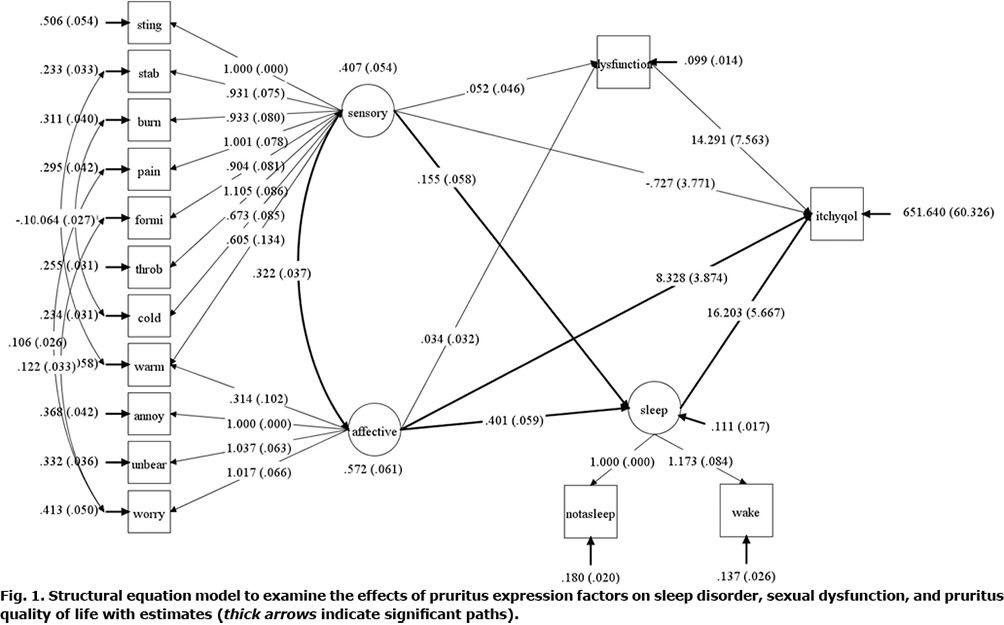
In addition to confirming the factor structure with psychometric properties, this study examined the associations among the factors of pruritus expression, sleep disturbance, sexual dysfunction, and ItchyQoL. As shown in Fig. 1, it was hypothesized that the factors of pruritus expression predict sleep disturbance, sexual dysfunction, and ItchyQoL, and that sleep disturbance and sexual dysfunction further predict ItchyQoL. In addition to assumption checks in EFA/CFA, they were also checked with additional items, sexual dysfunction, sleep disorder, ItchyQoL, which were satisfactory, except for the kurtosis of sexual dysfunction (Table SI).

Model evaluation
In both CFA and structural equation modelling (SEM), we applied estimates with χ2 test of exact-fit hypothesis test using robust maximum likelihood estimates, through the Maximum likelihood parameter estimates with standard errors and a chi-square test statistic option in Mplus 8 (25). The factor structure found was then evaluated by factor analysis and SEM models using approximate fit indices, including root mean square error of approximation (RMSEA), comparative fit index (CFI), and standardized root mean square residuals (SRMR) with the following criteria for “good fit”: RMSEA < 0.06, CFI > 0.95, and SRMR < 0.08 (26), and “adequate (or acceptable) fit” at 0.08> RMSEA > 0.05 and 0.95 > CFI > 0.90 (27, 28).
RESULTS
Demographics and pruritus characteristics
Of the 419 patients included in the study, 215 (51.3%) were male and 204 (48.7%) were female, with a mean age of 46.6 ± 17.4 years. The mean duration of pruritus between onset and first visit to the hospital was 2.9 ± 5.1 years. Regarding the distribution of pruritus, 212 patients (50.6%) had localized pruritus limited to a defined body area, whereas 207 (49.4%) had generalized pruritus over the entire body surface. With respect to skin lesions, 272 patients (65.9%) had inflamed skin lesions, 46 (11.1%) had chronic secondary scratch lesions, and 95 (23.0%) had clinically normal skin. The most common aetiologies of pruritus were dermatological causes (75.7%), including urticaria, atopic dermatitis, lichen simplex chronicus, prurigo nodularis, allergic contact dermatitis, seborrhoeic dermatitis, psoriasis, nummular eczema, xerotic eczema, drug eruption, and hand eczema, among others. Other aetiologies of pruritus included systemic, psychogenic, neurological, senile, and idiopathic pruritus (Table I).

Factors identified by pruritus descriptors via exploratory factor analysis/confirmatory factor analysis
Exploratory factor analysis. After searching for a factor structure from 1-factor to 3-factor for patient’s descriptions of pruritus, the 2-factor model was selected based on criteria of Kaiser rule (eigenvalue > 1.00) and parallel analysis (50 replications of empirical data, Fig S1). Of the 11 pruritus descriptors, 2 item clusters were identified in a clinically meaningful manner. These 2 clusters were named based on the similarities between the descriptors in each cluster. One cluster was thought to be a unique expression of one’s own pruritus, and the other cluster was thought to a complaint of psychological distress rather than a specific description of pruritus. Thus, we named the factors corresponding to the 2 clusters, as follows: factor I consisted of 7 pruritus descriptors: “stinging”, “stabbing”, “burning”, “painful”, “formication”, “throbbing”, and “cold.” Factor II consisted of 4 descriptors of “annoying”, “unbearable”, “worrisome”, and “warm.” This classification was attempted using the Eppendorf pruritus questionnaire published by Darsow et al. (29). They classified various descriptions of pruritus into sensory and affective dimensions. Utilizing their classifications to the results of this study, factor I was named “sensory pruritus” and factor II “affective pruritus”. Interestingly, warm sensations were included in the affective pruritus category, which was contrary to cold sensations. However, it should be noted that the indicator warm was equally well described by both sensory pruritus and affective pruritus on factor loadings of 0.479 and 0.519, respectively.
Confirmatory factor analysis. Based on the factor structure, CFA was fitted to the follow-up data of the 350 samples. The fit indices were within the acceptable ranges: CFI 0.940, SRMR 0.053, and RMSEA 0.077 and the factor loadings of CFA were listed in Table SII. Factor reliabilities were 0.882 for sensory pruritus and 0.842 for affective pruritus, which indicates that the factors measure the 2 factors consistently, whereas Cronbach’s alpha was 0.873 for sensory and 0.808 for affective pruritus. In contrast, retest reliability (intraclass correlation coefficients (ICC) between the baseline and follow-up measures) were 0.931 for sensory and 0.883 for affective. Based on the factor correlation (0.692), discriminant validity was confirmed. Factor loadings (Table II) were > 0.45, which confirmed the convergent validities for both factors.

Analysis of the association between each group and sleep disturbance, sexual dysfunction, and ItchyQoL within a structural equation modelling framework
The hypothesized model was fitted and its fit indices were in the good range: CFI 0.969, SRMR 0.036 and RMSEA 0.046. Sleep disturbance was significantly associated with ItchyQoL (b = 16.20, p = 0.004), and affective pruritus was significantly associated with ItchyQoL (b = 8.33, p = 0.032). However, sensory pruritus did not directly affect ItchyQoL (b = 0.727, p = 0.847). Affective pruritus also had a significant indirect effect on ItchyQoL (b = 6.498, p = 0.003). This significant indirect effect was due to the significant direct effect of affective pruritus on sleep disturbance (b = 0.401, p < 0.001). Sensory pruritus was also associated with sleep disturbance (b = 0.155, p = 0.007), but was less associated than affective pruritus. The indirect effects of sensory pruritus on ItchyQoL were not significant because of sleep disturbance (b = 2.518, p = 0.073) or sexual dysfunction (b = 0.745, p = 0.288). Sexual dysfunction was not significantly associated with pruritic descriptor factors or ItchyQoL scores. Although not significant, sexual dysfunction was marginally associated with ItchyQoL (b = 14.291, p = 0.059) (Fig. 1, Table III).

DISCUSSION
Pruritus is a multidimensional sensation that can be described in numerous ways. These unpleasant sensations can cause sleep disturbance or sexual dysfunction, leading to serious deterioration in quality of life (2, 11, 30). Most studies on chronic pruritus evaluation are closely related to the quality of life of patients, and focus on measuring the intensity of pruritus. The authors recently reported a high correlation between these unidimensional scales and quality of life, but this was lower than the correlation of multidimensional scales (i.e. ISS) with ItchyQoL (19). Although pruritus is the most important and common symptom experienced by dermatological patients, there is a lack of research and related information regarding the relationship between the quality of pruritus, sleep disorders, and quality of life in patients. The purpose of the current study was to group the interrelationships of various chronic pruritus-related descriptors due to dermatological or systemic diseases, rather than those of a specific disease or patient group. This study also aimed to analyse differences in sleep disorders, sexual dysfunction, and health-related quality of life in each group.
To our knowledge, this study is the first to perform a qualitative analysis of pruritus through a prospective, multicentre study, without being limited to specific diseases or age groups.
This study classified 11 pruritus descriptors as either sensory or affective pruritus. Affective pruritus directly and indirectly influences quality of life through sleep disorders. Sensory pruritus descriptors also affected sleep disorders, but had little direct or indirect effect on quality of life.
The warm sensation descriptor, even if it can be considered a sensory dimension, such as “cold”, had more affective implications, rather than being simply perceived as purely sensory. Linguistic and cultural contexts, such as how stressful situations are described as “fever-causing” in Korea, could have affected this. The current results differ from those of another study analysing the association between subjective descriptors and ItchyQoL of 405 US veterans reporting chronic pruritus, wherein “hot” was classified as a sensory dimension (4). In addition, a Danish study that investigated the dimensions of pruritus in patients with psoriasis, burning, and stinging were classified as affective dimensions (8). This discrepancy between studies conducted in different countries means that linguistic and cultural differences in different countries need to be accounted for when interpreting results.
Few studies have evaluated the quality of pruritus in a specific disease or group of patients. Yosipovitch et al. analysed the relationship between pruritus quality and intensity in patients with uraemic pruritus, but did not consider their skin diseases (2). They used a specific questionnaire constructed for the evaluation and measurement of pruritus. The most common sensory descriptors of pruritus were crawling (40%), stinging (39%), and tickling (15%). The most common affective descriptors were bothersome (80%), annoying (68%), and unbearable (33%). A correlation was noted between the affective score and VAS score during the worst state of the pruritus. A weak correlation was noted between the sensory and affective scores. No correlation was noted between the sensory and VAS scores.
A study of 40 patients with psoriasis assessed the subjective dimensions of pruritus and their association with psychological symptoms and quality of life (8). The patients completed a structured pruritus questionnaire, with a scale containing the descriptors, together with measures of depression, distress, sleep quality, and ItchyQoL. Factor analysis of the descriptors confirmed both the affective and sensory pruritus severity dimensions. Multivariate statistical analysis, controlling for age, sex, disease duration, and severity, showed that the severity of affective, but not sensory, pruritus was a significant predictor of depressive symptoms, global distress, sleep impairment, and ItchyQoL. This study showed that pruritus is multidimensional, and that the affective dimension may be the most important predictor of pruritus-related psychological morbidity. Although a small number of patients with psoriasis were studied, the results were similar to those of the current study.
Study limitations
This study has some limitations. First, selection bias should be considered, because patients visiting university hospitals may have felt anxious due to pruritus refractory to standard therapies. Secondly, this study enrolled only participants from Korean hospitals. It may be difficult to apply these results to other countries because the subjective descriptors for pruritus can have affective implications depending on linguistic and cultural differences. Thirdly, although baseline data were utilized for EFA and follow-up data for CFA, it would be more valid to use 2 different samples to identify and confirm the factor structure of the pruritus descriptors.
Conclusion
This study identified how patients described pruritus and its association with sleep disorders, sexual dysfunction, and poor quality of life. Patients who described their pruritus through affective pruritus descriptors experienced more severe sleep disturbances and had a poorer quality of life. In clinical settings, these results may improve the assessment of pruritus, which is usually performed by determining the degree of pruritus. Therefore, we believe that a patient’s description of pruritus contains information regarding sleep disorders or deterioration in health-related quality of life. These findings can be used to study the mechanism by which patients accept, interpret, and express the highly subjective symptoms of pruritus in the brain.
ACKNOWLEDGEMENTS
This work was supported by the Biomedical Research Institute grant, Kyungpook National University Hospital (2021). This research was also supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI15C001).
Reviewed and approved by the Kyungpook National University Hospital Institutional Review Board (IRB number: KNUH 2021-01-036).
The authors have no conflicts of interest to declare.
REFERENCES
- Yonova D. Pruritus in certain internal diseases. Hippokratia 2007; 11: 67–71.
- Yosipovitch G, Zucker I, Boner G, Gafter U, Shapira Y, David M. A questionnaire for the assessment of pruritus: validation in uremic patients. Acta Derm Venereol 2001; 81: 108–111.
- Paul J. Descriptors for itch related to chronic wounds. Wounds 2018; 30: 4–9.
- Rolader R, DeGrazia TM, Zhang C, Yosipovitch G, Chen SC, Yeung H. Factor analysis of subjective descriptors of chronic pruritus and association with quality of life: a cross-sectional survey. J Am Acad Dermatol 2020; 82: 1519–1521.
- Brenaut E, Garlantezec R, Talour K, Misery L. Itch characteristics in five dermatoses: non-atopic eczema, atopic dermatitis, urticaria, psoriasis and scabies. Acta Derm Venereol 2013; 93: 573–574.
- Huet F, Faffa MS, Poizeau F, Merhand S, Misery L, Brenaut E. Characteristics of pruritus in relation to self-assessed severity of atopic dermatitis. Acta Derm Venereol 2019; 99: 279–283.
- Briand C, Gourier G, Poizeau F, Jelti L, Bachelerie M, Quéreux G, et al. Characteristics of pruritus in bullous pemphigoid and impact on quality of life: a prospective cohort study. Acta Derm Venereol 2020; 100: adv00320.
- Zachariae R, Zachariae CO, Lei U, Pedersen AF. Affective and sensory dimensions of pruritus severity: associations with psychological symptoms and quality of life in psoriasis patients. Acta Derm Venereol 2008; 88: 121–127.
- Lim YL, H. CY, Yosipovitch G, Greaves MW. Pruritus is a common and significant symptom of acne. J Eur Acad Dermatol Venereol 2008; 22: 1332–1336.
- Kini SP, DeLong LK, Veledar E, McKenzie-Brown AM, Schaufele M, Chen SC. The impact of pruritus on quality of life: the skin equivalent of pain. Arch Dermatol 2011; 147: 1153–1156.
- Warlich B, Fritz F, Osada N, Bruland P, Stumpf A, Schneider G, et al. Health-related quality of life in chronic pruritus: an analysis related to disease etiology, clinical skin conditions and itch intensity. Dermatology 2015; 231: 253–259.
- Erturk IE, Arican O, Omurlu IK, Sut N. Effect of the pruritus on the quality of life: a preliminary study. Ann Dermatol 2012; 24: 406–412.
- Kong TS, Han TY, Lee JH, Son SJ. Correlation between severity of atopic dermatitis and sleep quality in children and adults. Ann Dermatol 2016; 28: 321–326.
- Kaaz K, Szepietowski JC, Matusiak Ł. Influence of itch and pain on sleep quality in atopic dermatitis and psoriasis. Acta Derm Venereol 2019; 99: 175–180.
- Sommer R, Augustin M, Hilbring C, Ständer S, Hubo M, Hutt HJ, et al. Significance of chronic pruritus for intrapersonal burden and interpersonal experiences of stigmatization and sexuality in patients with psoriasis. J Eur Acad Dermatol Venereol 2021; 35: 1553–1561.
- Hawro T, Przybyłowicz K, Spindler M, Hawro M, Steć M, Altrichter S, et al. The characteristics and impact of pruritus in adult dermatology patients: a prospective, cross-sectional study. J Am Acad Dermatol 2021; 84: 691–700.
- Majeski CJ, Johnson JA, Davison SN, Lauzon CJ. Itch Severity Scale: a self-report instrument for the measurement of pruritus severity. Br J Dermatol 2007; 156: 667–673.
- Desai NS, Poindexter GB, Monthrope YM, Bendeck SE, Swerlick RA, Chen SC. A pilot quality-of-life instrument for pruritus. J Am Acad Dermatol 2008; 59: 234–244.
- Jang YH, Kim SM, Eun DH, Park KD, Park GH, Kim BS, et al. Validity and reliability of itch assessment scales for chronic pruritus in adults: a prospective multicenter study. J Am Acad Dermatol 2020; 82: 80–86.
- Pett MA, Lackey NR, Sullivan JJ. Making sense of factor analysis: the use of factor analysis for instrument development in health care research. Sage, Thousand Oaks, CA; 2003.
- Brown TA. Confirmatory factor analysis for applied research. Guilford Publications, New York, NY; 2015.
- Furr M. Scale construction and psychometrics for social and personality psychology. Sage Publications Ltd, Los Angeles, CA; 2011.
- Raykov T. Behavioral scale reliability and measurement invariance evaluation using latent variable modeling. Behavior Therapy 2004; 35: 299–331.
- Nunnally JC. Psychometric theory 3E: Tata McGraw-hill education; 1994.
- Muthén B, Muthén L. Mplus. Chapman and Hall/CRC, Los Angeles, CA; 2017.
- Hu Lt, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equat Model 1999; 6: 1–55.
- Browne MW, Cudeck R. Alternative ways of assessing model fit. In: Bollen KA, Long JS, editors. Testing structural equation models: Newbury Park, CA: Sage; 1993: p. 136–162.
- Bentler PM. Comparative fit indexes in structural models. Psychol Bull 1990; 107: 238–246.
- Darsow U, Mautner V, Bromm B, Scharein E, Ring J. The Eppendorf Pruritus Questionnaire. Hautarzt 1997; 48: 730–733.
- Andrade A, Kuah CY, Martin-Lopez JE, Chua S, Shpadaruk V, Sanclemente G, et al. Interventions for chronic pruritus of unknown origin. Cochrane Database Syst Rev 2020; 1: Cd013128.