The majority of cases of atopic dermatitis (AD) in children under 6 years of age are mild (mild AD: 67%, moderate AD 26%, severe AD 7%) (1). AD in young children (age 6–12 years) and adolescents (age 13–17 years) has been the subject of several recent evaluations (2, 3). However, the impact of AD on parents of very young atopic children under 6 years of age has not been evaluated recently. The aim of this study was to evaluate this impact.
METHODS AND RESULTS
A total of 545 parents of children with physician-confirmed AD aged 0.5–6 years were recruited from a representative sample of the French population (identification of parents with a child with eczema among a representative sample constructed according to the quota method). Clinical severity was self-assessed by the child’s parent using the Patient-Oriented Eczema Measure (POEM). The impact on families was assessed by 3 validated questionnaires: the EuroQol 5 Dimensions 5 levels (EQ5-D, not specific to dermatoses) (4), the Dermatitis Family Impact (DFI: specific to dermatoses, but not to AD) and the Atopy Burden Scale – Family (ABS-F, specific to AD).
To complete this evaluation, the out-of-pocket expenses (OOP-E) for the parents for care of their children were evaluated. Severity of AD, assessed by POEM, identified mild AD in 347 (64%), moderate AD in 165 (30%) and severe AD in 33 (6%) patients. Regardless of the tool used, whether or not it was specific to dermatosis, and whether or not it was specific to AD, a positive gradient was observed according to the severity of AD. The mean utility function score (TTO) based on the EQ-5D-3 L, mean parental DFI scores, and mean ABS-F scores are described in Table I. The impact felt by the parents on activities of daily living is also reported in Table I. The more severe the eczema, the greater the impact.
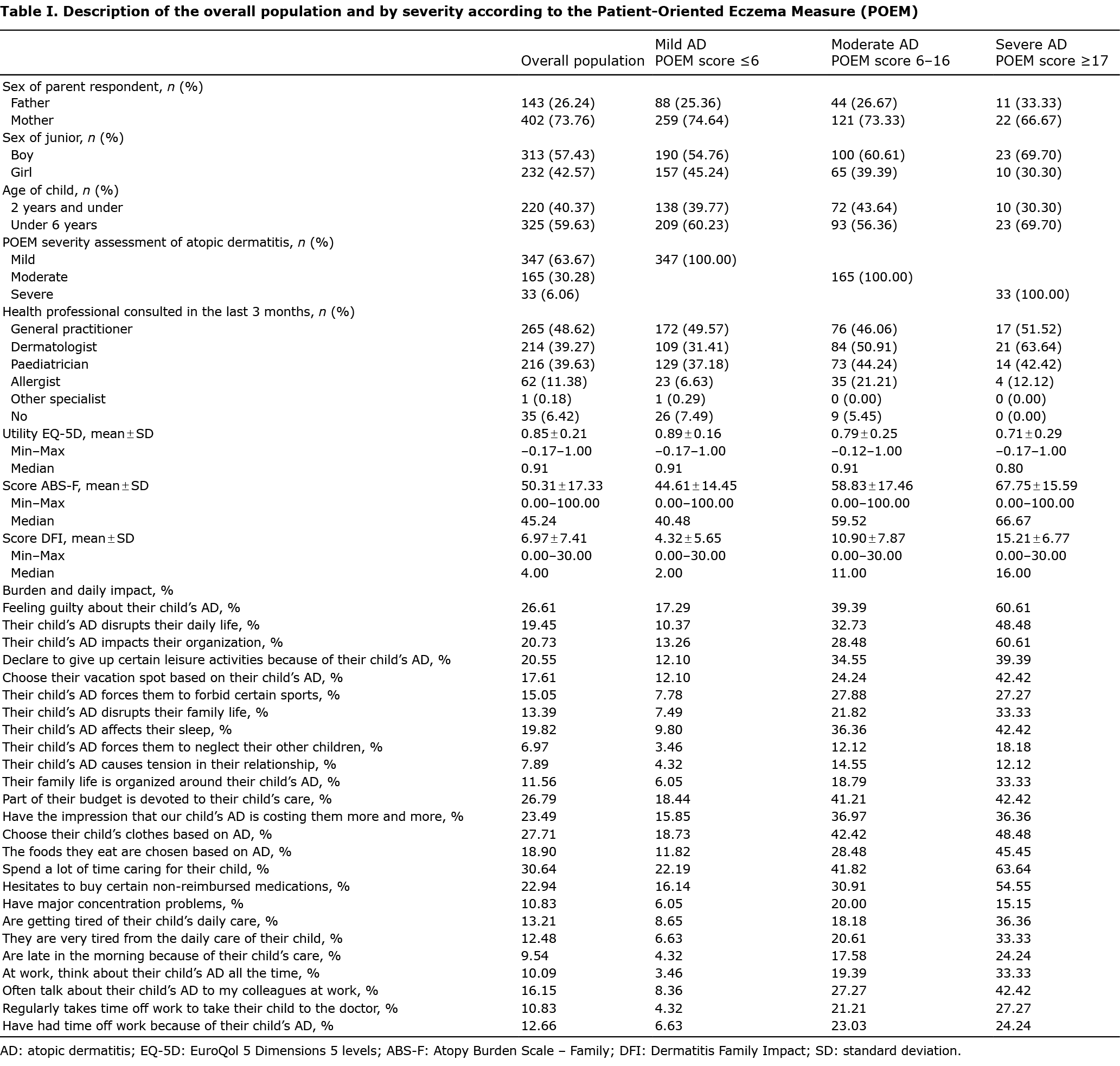
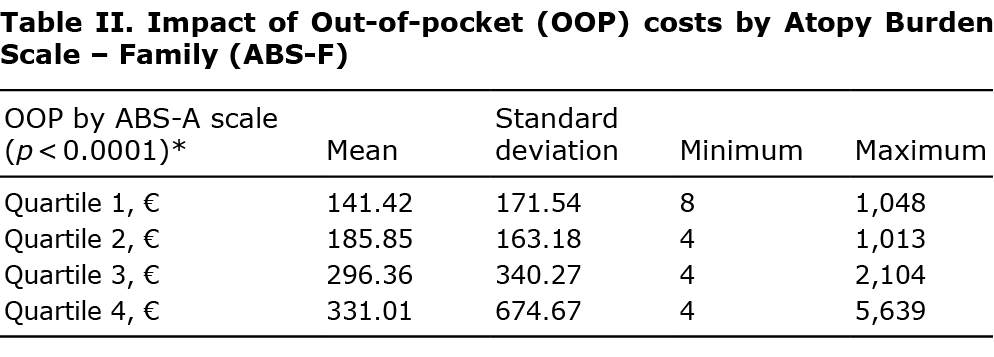
The mean annual OOP-E for the study population was 238 ± 388€. An evaluation of the mean OOP-E was carried out by considering the population of parents according to the different quartiles of the ABS-F score (the first quartile describes the population with the least severe burden, and the last describes the population with the most severe burden). These results clearly show that the OOP-E increases with the severity of the parent’s burden assessed through the ABS-A score: the more intense the burden, the higher the patient’s OOP-E: 141 ± 172€ for the 1st quartile, then, respectively, 186 ± 163€; 296 ± 340€, 331 ± 675€ for the 2nd, 3rd and 4th quartiles (Table II). A linear regression was conducted to determine to what extent the burden score can influence the family’s OOP-E: 10 ABS-F points increase the OOP-E by 50€. When the age and sex of the child, the income of the parents and the burden were included in a linear model, it emerged that only the burden was statistically related to the OOP-E.
DISCUSSION
This study on the impact of AD in parents of children under 6 years of age is, in terms of prevalence regarding severity levels, in line with published studies. The current study confirms that the impact on the family is all the more important, as the AD is severe regardless of the tool used. With a significant difference between the groups whether the tool used was specific to the field of dermatology (DFI for example) or specific to atopic dermatitis (ABS-F for example). Moreover, this is the first study to show a link between burden and OOP-E, highlighting that 10 points of burden generate 50€ of OOP-E. This high OOP-E could be explained by the fact that the French healthcare system does not pay enough for consultations with psychologists and almost none for emollients.
ACKNOWLEDGEMENTS
This study received funding from Sanofi.
The authors have no conflicts of interest to declare.
REFERENCES
- Silverberg JI, Simpson EL. Association between severe eczema in children and multiple comorbid conditions and increased healthcare utilization. Pediatr Allergy Immunol 2013; 24: 476–486.
- Ezzedine K, Shourick J, Merhand S, Sampogna F, Taieb C. Perceived clinical severity of atopic dermatitis in adolescents: comparison between patients’ and parents’ evaluation. J Am Acad Dermatol 2021; 84: 164–165.
- Ezzedine K, Shourick J, Merhand S, Sampogna F, Taïeb C. Impact of atopic dermatitis in adolescents and their parents: a French study. Acta Derm Venereol 2020; 100: adv00294.
- Pickard AS, Kohlmann T, Janssen MF, Bonsel G, Rosenbloom S, Cella D. Evaluating equivalency between response systems: application of the Rasch model to a 3-level and 5-level EQ-5D. Med Care 2007; 45: 812–819.