Xerosis in Patients with Type 2 Diabetes: An Italian Multicentre Study
Luca Stingeni1, Marta Tramontana1, Lorenzo Cordera2,3, Michela Castello4 and Aurora Parodi5; on behalf of the Italian Diabetix Group*
1Section of Dermatology, Department of Medicine and Surgery, University of Perugia, IT-61000 Perugia, 2Section of Endocrinology, School of Medicine, University of Genoa, 3Diabetes Unit, Ospedale-Policlinico San Martino IRCCS Genoa, 4Dermatology specialist, Pavia, and 5Dermatology Clinic, DiSSal University of Genoa, Ospedale-Policlinico San Martino IRCCS, Genoa, Italy. E-mail: luca.stingeni@unipg.it
Accepted Sep 29, 2021; Epub ahead of print Sep 29, 2021
doi: 10.2340/actadv.v101.263

Xerosis is a very common skin disorder in patients with type 2 diabetes mellitus (T2DM). Xerosis is characterized by scaling and flaking, can affect any area, and is often associated with a high burden of disease (1, 2), being a relevant discomfort factor for patients. Among the various skin disorders occurring in diabetic patients (e.g. acanthosis nigricans, necrobiosis lipoidica, granuloma annulare, vitiligo, lichen planus, diabetic foot syndrome, callosities, pruritus, and fungal and bacterial infections), a high correlation between xerosis and diabetic hand syndrome and keratosis pilaris has been described (3). As xerosis is associated with increased transepidermal water loss, documenting skin barrier damage, correct management is mandatory in order to preserve the protective barrier function of the skin (4, 5). Although quantification tools for xerosis are rarely used in clinical practice, treatment for xerosis can limit skin complications, such as cracks, fissures, superinfections, and ulcers (1). Xerosis is common and disabling, and is often underdiagnosed and undertreated by physicians, and thus left to the care of the patients themselves.
MATERIALS AND METHODS
An observational, prospective, non-interventional, multicentre study was performed on a cohort of Italian patients with T2DM between April 2017 and January 2018. The aim of the study was to assess the prevalence of xerosis in patients with T2DM, defining its severity and exposure to risk factors (e.g. sun exposure, employment).
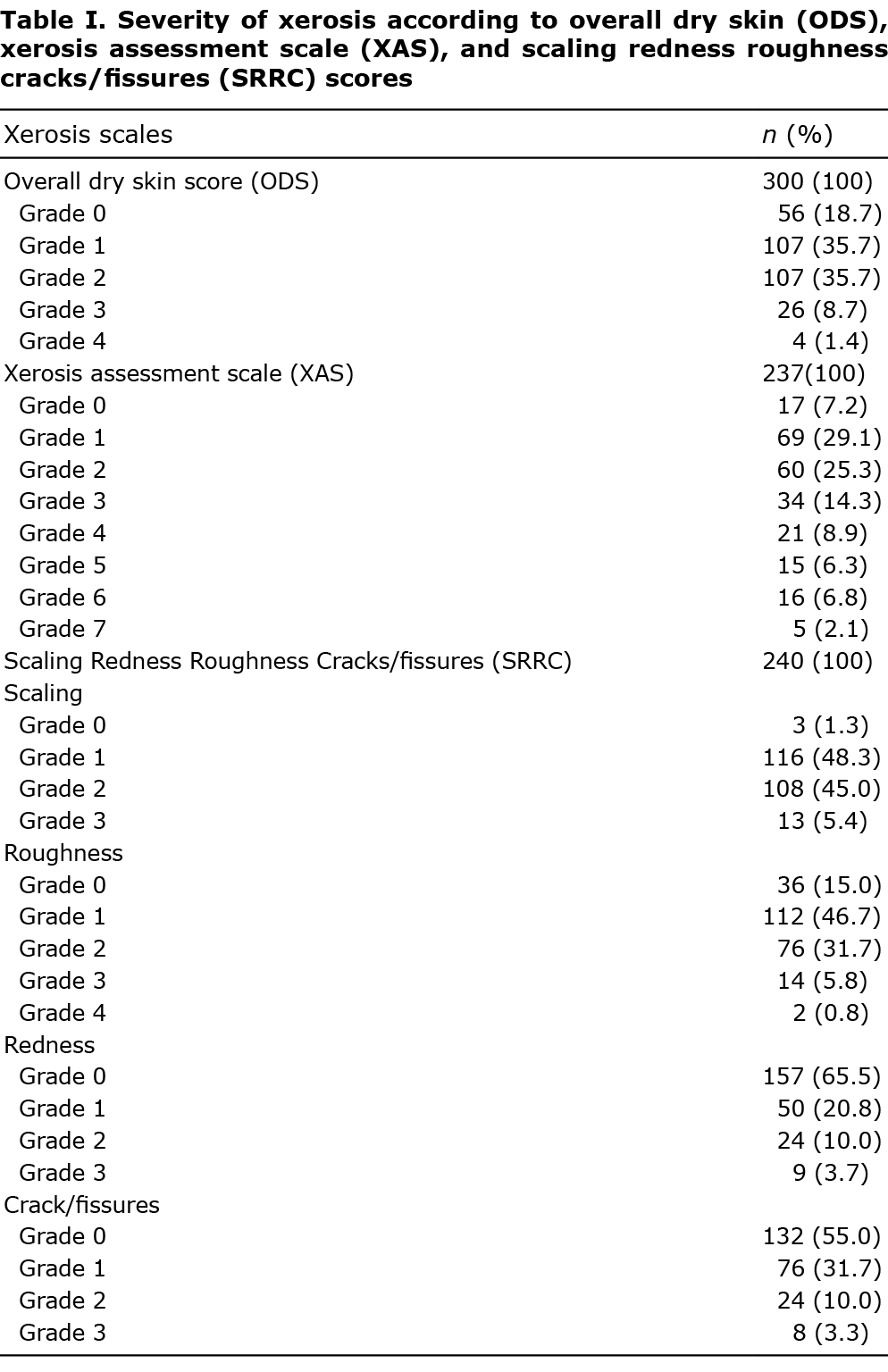
Patients aged ≥ 18 years affected by T2DM and followed up for ≥ 1 year consecutively attending the outpatient Clinics of Diabetology and Dermatology at 16 Centers homogeneously distributed in Italy, were enrolled. Presence of xerosis was diagnosed by a dermatologist within the centre for diabetes care, performed as part of the diabetological follow-up. Xerosis was scored by Overall Dry Skin (ODS), Xerosis Assessment Scale (XAS), Scaling Redness Roughness Cracks/fissures (SRRC) and a 10-cm VAS scale for itching (6–9). Scales are reported in the supplementary information.
The study was approved by the local ethics committee of each participating centre and written informed consent was provided by patients before any study procedures, in accordance with the Declaration of Helsinki.
The results are presented as percentages or means ± standard deviation (SD). Logistic regression and Student’s t-test were used to identify potential xerosis-related factors. Subjects without xerosis represented the comparison group for the analysis.
RESULTS
Overall, 327 subjects were enrolled (168 (51.4%) male), mean age 68.5 ± 11.0 years (range 25–95). Mean body mass index (BMI) was 29.5 ± 5.6 kg/cm2 (range 18.3–61.0). Mean time from diagnosis of T2DM was 14.1 ± 10 years (range 0–51.3). Mean level of HbA1c was 7.46 ± 1.6% (range 3.6–19.4%). Treatment for T2DM was currently prescribed to 288 (88.1%) subjects: biguanides were the most-used drug (212 subjects, 73.6%), followed by sulfonylurea (93, 32.3%) and dipeptidyl peptidase 4 inhibitors (67, 23.7%). Diabetes complications were present in 128 (39.1%) patients: angiopathy was found in 58 patients (45.3%), peripheral neuropathy in 46 (35.9%), retinopathy in 55 (43.0%), and nephropathy in 37 (28.9%).
ODS score was evaluated in 300 patients and xerosis was diagnosed in 244 (81.3%). Xerosis involved feet in 220 (90.2%), lower limbs in 204 (83.6%), upper limbs in 101 (41.4%), trunk in 77 (31.6%) and hands in 59 (24.2%). Severity scores for xerosis according to SRRC are shown in Table I. According to ODS and XAS scores, obtained in 314 patients, severe xerosis (either ODS ≥ 3 or XAS ≥ 5) was observed in 55 (17.5%) patients. The mean SRRC score was 4.0 ± 2.4 (range 0–13). Among patients with xerosis, itching was reported in 168 (68.9%); the mean VAS score for itching was 2.48 ± 2.37 (range 0–10).
Sex and age of patients were not statistically associated with presence of xerosis in this study population. Patients aged ≤ 65 years had a reduced risk of severe xerosis compared with older diabetic patients (odds ratio (OR) 0.41, 95% confidence interval (95% CI) 0.22–0.75; p = 0.004). No statistically significant correlation between xerosis and common diabetes-related complications was observed, particularly with diabetic neuropathy, even if patients with angiopathy had a higher risk of severe xerosis compared with patients without angiopathy (OR 1.38, 95% CI 1.16–13.56; p = 0.028). In contrast, no correlation with the presence of xerosis was found for BMI, duration of diabetes, HbA1c level and the overall presence of diabetes complications. Subjects with severe xerosis (n = 23) showed excessive sun exposure (OR 4.97; 95% CI 1.25–19.81; p = 0.02) and were employers in cleaning companies (OR 12.42; 95% CI 1.31–118.02; p = 0.02).
DISCUSSION
T2DM is a common metabolic disease, characterized by several systemic complications. Keratinocytes and fibroblasts are damaged by chronic hyperglycaemia, as well as neurones, renal and endothelial cells (1). Thus, several skin disorders may occur in diabetic patients, of which xerosis is frequent in elderly patients (1), occurring in 26–44% of subjects with T2DM (1, 2).
This study documented a prevalence of xerosis that was considerably higher (81.3%) than reported in the literature (1, 2), although studies on the prevalence of xerosis in subjects with T2DM are rare. This discordance could be due to the lack of standardization of the tools used to evaluate xerosis and the different demographic features of studied populations. Notably, xerosis was severe in 17.5% of patients in the current study, especially in those aged ≥ 65 years. The correlation between severe xerosis and angiopathy observed in the current study was previously reported by Kamel et al. (10) in paediatric patients with T1DM, in whom xerosis was more frequent in patients with vasculopathic kidney damage, suggesting a relationship between cutaneous and extracutaneous complications in diabetic patients. Although the current study did not observe a significant correlation between xerosis and diabetic neuropathy, as reported by Yosipovitch et al. (3), further research is needed into this association.
The pathomechanism of xerosis in T2DM is not fully understood. It is well known that diabetic skin is characterized by a decrease in production of sebum and triglycerides from the sebaceous glands due to an impaired insulin signal (11, 12). During long-term hyperglycaemia, skin collagen deposits advanced glycated end products (AGE) and non-enzymatic glycosylation of other proteins, such as laminin and elastase (13), occurs. Alterations in diabetic skin are also due to abnormal, persistent cohesion between corneocytes, with impaired moisturization of the uppermost epidermal layers and altered skin barrier function (14). In fact, even in the absence of clinically apparent xerosis, a discrete “dry skin” feeling, potentially evolving in more severe xerosis, is common (1, 13).
Finally, the results of the current study suggest that some lifestyle habits and occupations, such as sun exposure and wet work, may increase the risk of xerosis in people with diabetes. Therefore, educational programmes proposed by dermatologists and diabetologists may help diabetic patients to prevent xerosis and its complications, thus reducing their overall burden of disease.
ACKNOWLEDGEMENTS
The study was approved by the ethics committee of each participating centre. Written informed consent was provided by patients before any study procedures, in accordance with the Declaration of Helsinki.
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Conflicts of interest and funding. LS acted as speaker or board member for Abbvie, Amgen, Eli-Lilly, Novartis, Sanofi, Leo Pharma, Janssen, outside the present study. MT has no competing interests. MC was employed in Pierre Fabre, Italy, when Diabetix Study was carried out. AP acted as speaker or consultant for Almirall, UCB, Novartis, Eli Lilly, LEO Pharma, Abbvie, Pfizer, Galderma, Amgen, Pierre Fabre, and Sanofy.
Pierre Fabre, Italy, gave a grant for Diabetix study and funded the editorial assistance provided by Aashni Shah (Polistudium SRL, Milan, Italy).
REFERENCES
- Lima AL, Illing T, Schliemann S, Elsner P. Cutaneous manifestations of diabetes mellitus: a review. Am J Clin Dermatol 2017; 18: 541–553.
- Grossman AB. Clinical evaluation of 35% urea in a water–lipid-based foam containing lactic acid for treatment of mild-to-moderate xerosis of the foot. J Am Podiatr Med Assoc 2011; 101: 153–158.
- Yosipovitch G, Hodak E, Vardi P, Shraga I, Karp M, Sprecher E, David M. The prevalence of cutaneous manifestations in IDDM patients and their association with diabetes risk factors and microvascular complications. Diabetes Care 1998; 21: 506–509.
- Martini J, Huertas C, Turlier V, Saint-Martory C, Delarue A. Efficacy of an emollient cream in the treatment of xerosis in diabetic foot: a double-blind, randomized, vehicle-controlled clinical trial. J Eur Acad Dermatol Venereol 2017; 31: 743–747.
- Seité S, Khemis A, Rougier A, Ortonne JP. Importance of treatment of skin xerosis in diabetes. J Eur Acad Dermatol Venereol 2011; 25: 607–609.
- Pham HT, Exelbert L, Segal-Owens AC, Veves A. A prospective, randomized, controlled double-blind study of a moisturizer for xerosis of the feet in patients with diabetes. Ostomy Wound Manage 2002; 48: 30–36.
- Serup J. EEMCO guidance for the assessment of dry skin (xerosis) and ichthyosis: clinical scoring systems. Skin Res Technol 1995; 1: 109–114.
- Hashmi F, Nester C, Wright C, Newton V, Lam S. Characterising the biophysical properties of normal and hyperkeratotic foot skin. J Foot Ankle Res 2015; 8: 35.
- Reich A, Heisig M, Phan NQ, Taneda K, Takamori K, Takeuchi S, et al. Visual analogue scale: evaluation of the instrument for the assessment of pruritus. Acta Derm Venereol 2012; 92: 497–501.
- Kamel MI, Elhenawy YI, Saudi WM. Relation between cutaneous and extracutaneous complications in pediatric patients with type 1 diabetes. Dermatoendocrinol 2018; 10: e1467717.
- de Macedo GM, Nunes S, Barreto T. Skin disorders in diabetes mellitus: an epidemiology and physiopathology review. Diabetol Metab Syndr 2016; 8: 63.
- Sakai S, Kikuchi K, Satoh J, Tagami H, Inoue S. Functional properties of the stratum corneum in patients with diabetes mellitus: similarities to senile xerosis. Br J Dermatol 2005; 153: 319–323.
- Ye X, Tong Z, Dang Y, Tu Q, Weng Y, Liu J, Zhang Z. Effects of blood glucose fluctuation on skin biophysical properties, structure and antioxidant status in an animal model. Clin Exp Dermatol 2010; 35: 78–82.
- Piérard GE, Seité S, Hermanns-Lê T, Delvenne P, Scheen A, Piérard-Franchimont C. The skin landscape in diabetes mellitus. Focus on dermocosmetic management. Clin Cosmet Investig Dermatol 2013; 6: 127–135.