General anaesthesia could affect various immune responses, including Th1 and Th2 immunity, which might also affect cells that play an important role in the pathogenesis of atopic dermatitis. However, the relationship between general anaesthesia exposure and atopic dermatitis remains unknown. The aim of this study was to investigate the risk of developing atopic dermatitis after first exposure to general anaesthesia in the paediatric population (18 years or under). A retrospective cohort study, including those exposed (n = 7,681) and unexposed (n = 38,405; control participants) to general anaesthesia (1:5 ratio), was conducted using national sample cohort data from 2002 to 2015. All participants were followed up for 2 years after cohort entry. The 2-year cumulative incidences of atopic dermatitis in the exposed and unexposed groups were 2.3% and 2.2%, respectively. In the subgroup analysis by age, the cumulative incidence was not significantly different between these cohorts. The risks of atopic dermatitis were not significant in the exposed group in the univariate model (hazard ratio 1.05; confidence interval 0.88–1.24) and in the multivariate model, wherein all covariates were adjusted (adjusted hazard ratio, 1.03; 95% confidence interval 0.87–1.23). The results suggest that children’s exposure to general anaesthesia was not associated with increased or decreased risk of atopic dermatitis.
Key words: atopic dermatitis; cohort analysis; general anaesthesia; paediatric population.
Accepted Nov 1, 2022; Epub ahead of print Nov 1, 2022
Acta Derm Venereol 2022; 102: adv00813.
DOI: 10.2340/actadv.v102.2738
Corr: Jee Woong Choi, Department of Dermatology, Ajou University School of Medicine, Ajou University Hospital, 164, World Cup-ro, Yeongtong-gu, Suwon-si, Gyeonggi-do 16499, South Korea. E-mail: dermaboy@gmail.com
SIGNIFICANCE
Atopic dermatitis is one of the most common skin disorders in childhood, and general anaesthesia has a risk of developing potential complications in various organs and anaphylaxis and allergies. However, the relationship between exposure to general anaesthesia and atopic dermatitis remains unknown. The results of this study revealed that exposure to general anaesthesia was not associated with atopic dermatitis in the paediatric population.
INTRODUCTION
Atopic dermatitis (AD), which is one of the most common skin disorders in childhood, is a chronic, inflammatory skin disease that impairs the quality of life of affected children and families (1). AD affects 5–20% of children worldwide (2, 3), and its prevalence is 5.9%, 11.3%, and 14.6% in infants, preschool children, and school-age children, respectively (4). It is a multifactorial heterogeneous disorder, and patients with AD have an increased risk of various comorbid conditions, such as attention-deficit hyperactivity disorder, infection, autoimmune diseases, including vitiligo and alopecia areata, psychosocial disorders, and visual impairment (5–7). Furthermore, AD has a development risk of asthma and allergic rhinitis, called the atopic march, as the child grows (8).
General anaesthesia (GA) has a risk of developing potential complications in various organs and anaphylaxis and allergies (9–11). Although the exact mechanism remains unknown, a previous cohort study showed that GA exposure before 1 year of age was associated with a lower risk of developing AD (12). Also, GA could affect various immunological pathways, including Th1 and Th2 immunity, which might also affect cells that play an important role in the pathogenesis of AD (3, 13). However, to date, studies on GA and allergic diseases in the paediatric population are very limited and insufficient; hence, the relationship between AD and GA remains unclear.
The aim of this retrospective cohort study was to assess the risk of development of AD after the first exposure to GA in the paediatric population, using a nationwide sample cohort that was followed for up to 2 years.
MATERIALS AND METHODS
Data sources
This study was conducted using data from the Korean National Health Insurance Service (NHIS) national sample cohort from 2002 to 2015, comprising 2.2% of the total eligible Korean population (14). Random samples were representative of the total population. Diagnosis of the sample cohort was based on the International Classification of Diseases, Tenth Revision (ICD-10). The data contained encrypted personal information, diagnostic codes, prescribed drugs, and medical claims. All registered and claimed data of the sampled individuals constituted the longitudinal database. The validity and usefulness of the cohort data have been verified in previous studies (15–19).
Study population
Information on GA exposure was extracted from the sample cohort database and divided into intravenous injection, endotracheal tube, and mask anaesthesia. During GA, injectable anaesthetics were used in intravenous anaesthesia, and inhalational anaesthetics were used in mask and endotracheal anaesthesia. The total duration of GA was also calculated. To reduce selection bias, the first 2 years (2002 to 2003) were set as the washout period. To define a GA-exposed cohort, patients who were exposed to GA from 1 January 2004, were included, and those who were given any diagnostic codes of AD (L20 and its sub-classification codes) prior to undergoing the first recorded exposure to GA were excluded. Adult patients aged 19 years and over were also excluded from the analysis.
For the GA-exposed participants, observation began on the day of the first exposure (cohort entry date). Age group- (infant and toddler (0–2 years), preschool (3–6 years), school age (7–12 years), adolescence (13–18 years)), sex-, and parents’ income level- were matched to random controls who were never exposed to GA, who were identified in a 1:5 manner to obtain an unexposed control group. The matched controls entered the study on the same day that follow-up began for their GA-exposed counterparts. Both cohorts were followed from the cohort entry dates until the day the diagnostic code of AD was first given, death, emigration, the end of December 2015, or to the day 1 year after the cohort entry date, whichever came first. Since a 2-year washout period was set before the analysis, the maximum observation period was set as 2 years after the cohort entry date, as the effect of GA on AD occurrence would decrease over time.
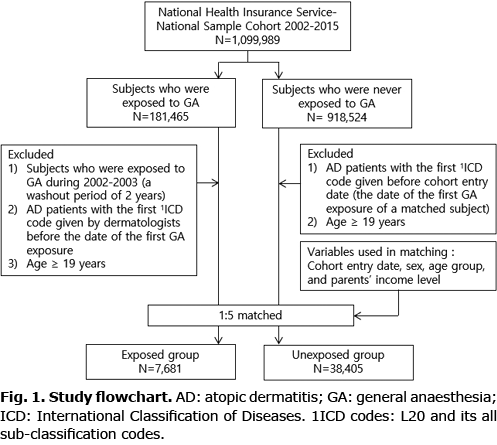
Among both cohorts, the participants with subsequent AD were defined as those who had at least 1 ICD-10 diagnostic code of AD given by board-certified dermatologists. To investigate the differences in its clinical features in both cohort groups, the patients with AD were divided into 3 groups according to the disease duration (≤ 6, 6–12, and > 12 months) and the duration of steroid or immunosuppressant use (no use, 1–30 days, and > 30 days). The primary independent variable of interest in this study was the first exposure to GA. The primary outcome was AD-free survival. A flowchart of the study is shown in Fig. 1.
Covariates
Demographic factors at the cohort entry date were collected, and the Charlson–Deyo scores were calculated according to a previous method (20). Data on prior exposure to regional anaesthesia, including spinal and epidural anaesthesia, were also collected. Pre-existing comorbid disorders, including systemic comorbidities (hypertension, diabetes, dyslipidaemia, and thyroid disorders), allergic comorbidities (angioedema or anaphylaxis, allergic rhinitis, asthma, and urticaria), immune-mediated dermatological comorbidities (lupus erythematosus, vitiligo, psoriasis, and alopecia areata), overall psychological disorders confirmed by psychiatrists, and overall malignancies were investigated using the corresponding ICD-10 codes in the diagnosis field.
Ethical statement
This study design was reviewed by the institutional review board of Ajou University Hospital (AJIRB-MED-EXP-20-050), and informed consent was waived by the institutional review board owing to the retrospective nature of the study.
Statistical analysis
Descriptive statistics were used to describe the baseline characteristics of the GA exposed and unexposed cohorts. The χ2 test or Fisher’s exact test was carried out to evaluate its statistical significance for categorical data. The Kaplan–Meier method was used to estimate survival curves for each cohort. A Cox proportional hazard regression model was used to obtain the hazard ratios (HRs) and 95% confidence intervals (95% CIs) using patients with no exposure as a reference, with and without adjusting for all baseline characteristics. The same model was used in the exposed cohort to assess the GA-related risk factors associated with AD. The Cox proportional hazard model assumption of proportionality was also assessed using Schoenfeld residuals. p-values < 0.05 were considered statistically significant. Statistical analyses were performed using SAS Enterprise Guide 7.1 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Demographic and clinical characteristics of the study population
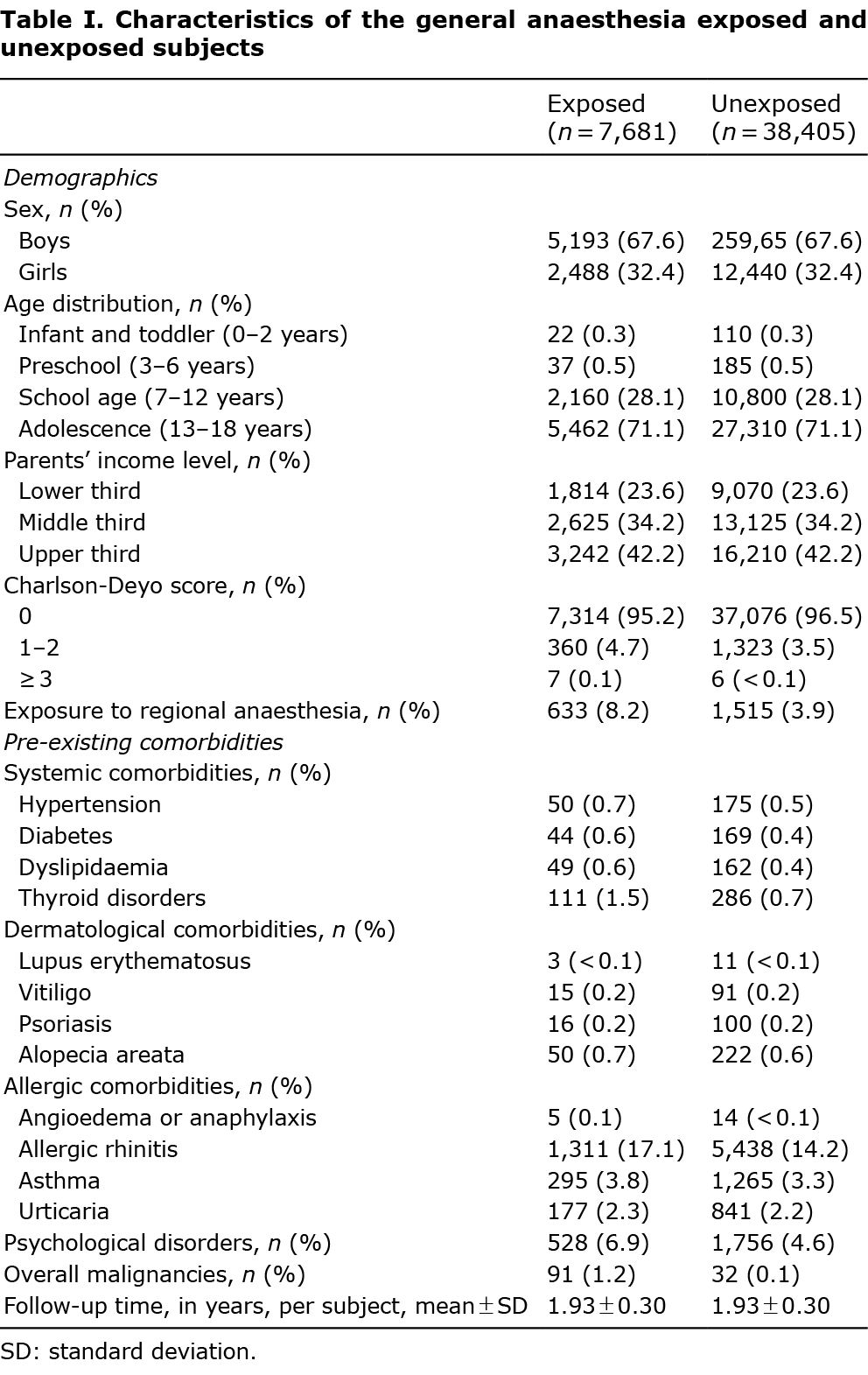
From January 2002 to December 2015, 7,681 GA-exposed patients and 38,405 unexposed patients, who satisfied the eligibility criteria, were identified from a representative sample cohort (Fig. 1). The demographics and characteristics of the exposed and unexposed patients are shown in Table I. All demographic features, except for the Carlson–Deyo scores, were similar between groups. The percentage of patients, who were exposed to regional anaesthesia or had pre-existing comorbidities, was higher in the exposed group than that in the unexposed group. All variables were included and adjusted as covariates in multivariate Cox regression models. The mean ± standard deviation follow-up time per patient of both groups was 1.93 ± 0.30 years.
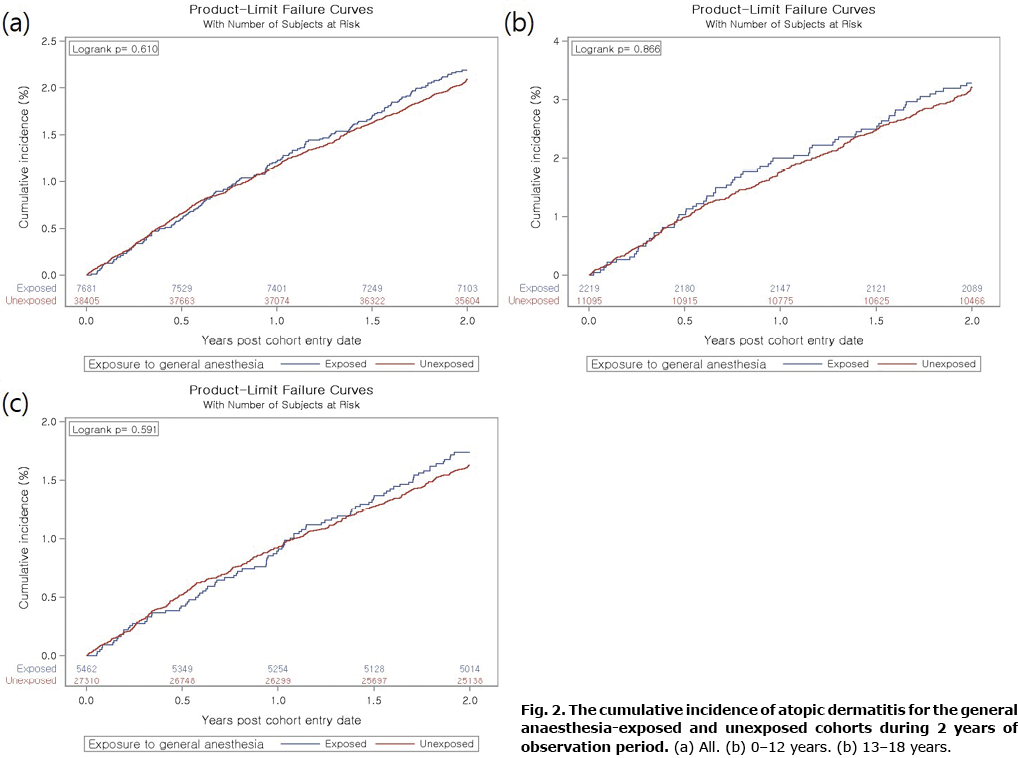
Cumulative incidence of atopic dermatitis in the general anaesthesia exposed and unexposed cohorts
During the follow-up period, the incidences of AD in the patients with or without GA were 11.1 and 10.6 per 1,000 person-years, respectively. The Kaplan–Meier curve showed no difference in the cumulative incidence of AD between the groups (log-rank p = 0.610). The 2-year cumulative incidence of AD in the GA and non-GA groups was 2.3% and 2.2%, respectively (Fig. 2a). In the subgroup analysis by age, the cumulative incidence was not significantly different in both cohorts (Fig. 2b, c).
Relative risk of atopic dermatitis in the general anaesthesia-exposed cohorts
The HRs for AD in the GA-exposed cohorts are summarized in Table II. Cox proportional hazard model assumption showed no significant deviation from time (p = 0.377) (Fig. S1). The risks of AD were not significant in the GA group in the univariate model (HR 1.05; 95% CI 0.88–1.24) and multivariate model wherein all covariates in Table I were adjusted (adjusted HR 1.03; 95% CI 0.87–1.23). Furthermore, when Cox regression analysis was performed in the GA group, there was no significant difference in the risk of AD depending on the type of GA and surgery (cancer vs non-cancer surgery), even after adjusting for baseline covariates. The duration of GA was not associated with the occurrence of AD.
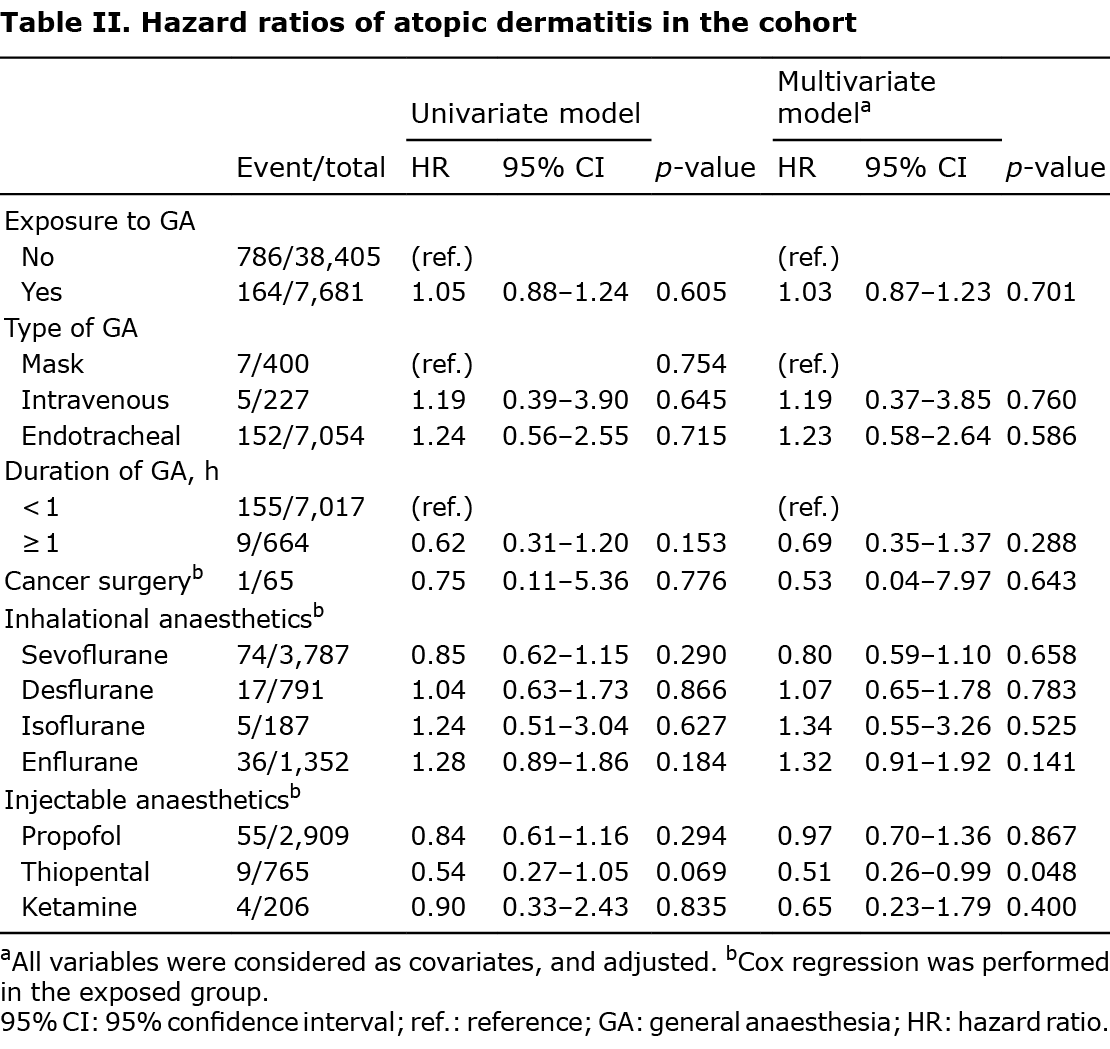
The risk of AD was not statistically significant according to the type of inhalational anaesthetics. Among the injectable anaesthetics, except for thiopental, there was no significant association with the risk of AD.
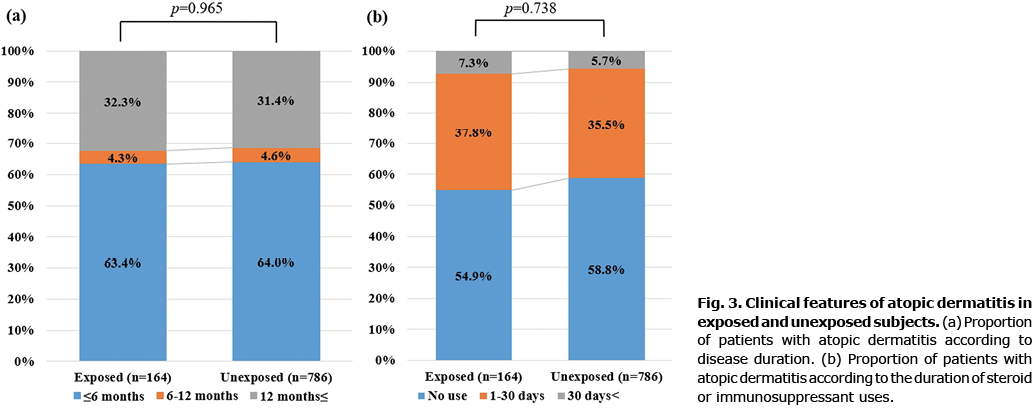
Clinical features of atopic dermatitis in general anaesthesia exposed and unexposed patients
The proportion of patients with AD was analysed according to disease duration and frequency of steroid or immunosuppressant use during the study period. In the GA group, the proportion of patients with AD who persisted for more than 6 months (Fig. 3a) or the proportion of patients using systemic steroids and immunosuppressants (Fig. 3b) was higher than those in the non-GA group; however, this was not statistically significant.
Sensitivity analysis
If patients with AD were more strictly defined as those who had at least ICD-10 diagnostic code of AD given by board-certified dermatologists twice, the HR on the AD occurrence in the GA group was not significant using univariate analysis (HR 1.04; 95% CI 0.83–1.30; p = 0.719). Similarly, in multivariate analysis with adjusted baseline characteristics, the risk of AD in the GA group was not significantly higher than that in the non-GA group (HR 1.04; 95% CI 0.83–1.30; p = 0.742) (data not shown). If patients with AD were defined as those who had at least ICD-10 diagnostic code of AD given by board-certified dermatologists 3 times, the GA exposure did not increase the risk of AD development both in the univariate (HR 0.98; 95% CI 0.75–1.28; p = 0.883) and multivariate analysis (adjusted HR 0.97; 95% CI 0.74–1.30; p = 0.839) (data not shown).
DISCUSSION
The number of patients undergoing GA in the paediatric population is increasing (21, 22), and thus interest in whether GA could be a risk factor for various diseases has increased (12, 23–26). In particular, previous studies revealed that exposure to GA in early childhood and multiple exposures increased the risk of developmental delay and attention deficit hyperactivity disorder (25, 26). Conversely, autistic disorders or learning disabilities were not associated with a single exposure to GA (24, 26). Also, it has been reported that exposure to GA in early life reduced the risk of asthma, allergic rhinitis, and AD (12).
The results of the current study revealed that exposure to GA was not associated with AD in the paediatric population. It was found that only 1 study has been conducted on the association between GA and AD in the recently conducted systematic review and meta-analysis of risk factors for AD (27). The paediatric population was classified into 3 groups: 0–2, 2–12, and 12–18 years (28). Also, the distribution of the lesions and immunological composition may differ according to age groups (29). Furthermore, at birth, most T cells are naive and developing gradually into memory subsets (29–31), and high 2 with low or unaltered 1 signals in blood were reported during the 0–2 years group. Kuo et al. reported that exposure to GA before 1 year of age reduced the risk of AD, since GA might provide a protective effect, promote inflammatory 1 responses, and decrease 2 immunity (12). If GA is effective in protecting against AD, the risk of developing AD decreases with age. However, in a subgroup analysis of the current study investigating the risk of AD in relation to age, results for GA exposure were consistent and not associated with AD. Based on the current results, we thought that GA might have a negligible effect on the risk of developing AD. Furthermore, GA might cause immunological changes during or immediately after surgery; however, it will be temporary and not persist to a significant extent in the general population (32). Therefore, temporary changes in immune responses were thought to induce a different response from the pathophysiology of AD as a chronic heterogeneous disease in children, and GA may not act as a protective or aggravating factor for the development of AD.
The current study found no significant association with the risk of AD according to anaesthetic use, except for thiopental. After adjusting for baseline covariates, thiopental significantly decreased the risk of AD, which might be related to an immune response mediated by thiopental that was distinct from other intravenous anaesthetics (33). A previous study revealed that thiopental reduced 2-stimulating IL-4 (34). Although the 2 immune response is important in the pathophysiology of AD, it is difficult to explain this with just 1 aspect, since AD is a heterogeneous disease. Therefore, it is difficult to conclude the existence of a direct relationship between thiopental and risk of AD; hence, further research is necessary.
The strength of the current study is that it is a nationwide population-based cohort study to investigate the risk of AD in paediatric participants exposed to GA, with a broader age range compared with previous studies. However, this study had several limitations. First, it was not possible to consider and adjust all risk factors for AD, such as genetic and environmental risk factors. Secondly, with the retrospective study design, this study could not measure the change in immunological effects according to GA. Thirdly, the number included in the cohort was relatively small in the age group of infants and toddlers.
Overall, whether GA could be a factor influencing AD requires careful interpretation, and the current study supported that GA was not associated with the development of AD in the paediatric population. Based on the results of this population-based, retrospective matched cohort study, it was concluded that exposure of children to GA was not associated with increased or decreased risk of AD. Therefore, GA in children was considered safe regarding the development of AD.
ACKNOWLEDGEMENTS
This study used the sample cohort data released by the NHIS. Only the authors were responsible for the content and writing of this manuscript. This study was supported by a 2020 Amorepacific grant.
This study design was reviewed by the institutional review board of Ajou University Hospital (AJIRB-MED-EXP-20-050).
The authors have no conflicts of interest to declare.
REFERENCES
- Yang EJ, Beck KM, Sekhon S, Bhutani T, Koo J. The impact of pediatric atopic dermatitis on families: a review. Pediatr Dermatol 2019; 36: 66–71.
- Kantor R, Thyssen J, Paller A, Silverberg J. Atopic dermatitis, atopic eczema, or eczema? A systematic review, meta-analysis, and recommendation for uniform use of ‘atopic dermatitis’. Allergy 2016; 71: 1480–1485.
- Çetinkaya PG, Şahiner ÜM. Childhood atopic dermatitis: current developments, treatment approaches, and future expectations. Turk J Med Sci 2019; 49: 963–984.
- Ha J, Lee SW, Yon DK. Ten-Year trends and prevalence of asthma, allergic rhinitis, and atopic dermatitis among the Korean population, 2008–2017. Clin Exp Pediatr 2020; 63: 278.
- Pols DH, Bohnen AM, Nielen MM, Korevaar JC, Bindels PJ. Risks for comorbidity in children with atopic disorders: an observational study in Dutch general practices. BMJ open 2017; 7: e018091.
- Gilaberte Y, Pérez-Gilaberte JB, Poblador-Plou B, Bliek-Bueno K, Gimeno-Miguel A, Prados-Torres A. Prevalence and comorbidity of atopic dermatitis in children: a large-scale population study based on real-world data. J Clin Med 2020; 9: 1632.
- Huang AH, Roh YS, Sutaria N, Choi J, Williams KA, Canner JK, et al. Real-world comorbidities of atopic dermatitis in the pediatric ambulatory population in the United States. J Am Acad Dermatol 2021; 85: 893–900.
- Dharmage SC, Lowe A, Matheson MC, Burgess J, Allen K, Abramson MJ. Atopic dermatitis and the atopic march revisited. Allergy 2014; 69: 17–27.
- Harris M, Chung F. Complications of general anesthesia. Clin Plast Surg 2013; 40: 503–513.
- Stepanovic B, Sommerfield D, Lucas M, von Ungern-Sternberg BS. An update on allergy and anaphylaxis in pediatric anesthesia. Paediatr Anaesth 2019; 29: 892–900.
- Kim JC, Kim DC, Choi YW, Lee ES, Choi JW. Association of chronic spontaneous urticaria with the first exposure to general anaesthesia. Clin Exp Allergy 2022; 52: 990–993.
- Kuo H-C, Yang Y-L, Ho S-C, Guo MM-H, Jiang J-H, Huang Y-H. General anesthesia exposure in early life reduces the risk of allergic diseases: a nationwide population-based cohort study. Medicine 2016; 95: e4269.
- Palacios AS, Ponce MO, Pérez AR, Medina FS, Marrero JG. Modification of mediators of immune reaction after general anaesthesia. Allergol Immunopathol (Madr) 2004; 32: 352–360.
- Lee J, Lee JS, Park SH, Shin SA, Kim K. Cohort profile: The National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int J Epidemiol 2017; 46: e15.
- Yoon J, Choi JW. Primary cicatricial alopecia in a single-race Asian population: a 10-year nationwide population-based study in South Korea. J Dermatol 2018; 45: 1306–1311.
- Soh BW, Kim SM, Kim YC, Choi GS, Choi JW. Increasing prevalence of alopecia areata in South Korea. J Dermatol 2019; 46: e331–e332.
- Kim JC, Lee ES, Choi JW. Impact of alopecia areata on psychiatric disorders: a retrospective cohort study. J Am Acad Dermatol 2020; 82: 484–486.
- Kim JC, Choi JW. Impact of alopecia areata on subsequent pregnancy rate: a retrospective cohort study. Australas J Dermatol 2021; 62: e121–e123.
- Lee H, Kim YC, Choi JW. Alopecia areata is not a risk factor for heart diseases: a 10-year retrospective cohort study. PLoS One 2021; 16: e0250216.
- Sundararajan V, Quan H, Halfon P, Fushimi K, Luthi JC, Burnand B, et al. Cross-national comparative performance of three versions of the ICD-10 Charlson index. Med Care 2007; 45: 1210–1215.
- Rabbitts JA, Groenewald CB, Moriarty JP, Flick R. Epidemiology of ambulatory anesthesia for children in the United States: 2006 and 1996. Anesth Analg 2010; 111: 1011–1015.
- Tzong KY, Han S, Roh A, Ing C. Epidemiology of pediatric surgical admissions in US children: data from the HCUP kids inpatient database. J Neurosurg Anesthesiol 2012; 24: 391–395.
- von Ungern-Sternberg BS, Boda K, Chambers NA, Rebmann C, Johnson C, Sly PD, et al. Risk assessment for respiratory complications in paediatric anaesthesia: a prospective cohort study. The Lancet 2010; 376: 773–783.
- Ko W-R, Huang J-Y, Chiang Y-C, Nfor ON, Ko P-C, Jan S-R, et al. Risk of autistic disorder after exposure to general anaesthesia and surgery: a nationwide, retrospective matched cohort study. Eur J Anaesthesiol 2015; 32: 303–310.
- Tsai C-J, Lee CT-C, Liang SH-Y, Tsai P-J, Chen VC-H, Gossop M. Risk of ADHD after multiple exposures to general anesthesia: a nationwide retrospective cohort study. J Atten Disord 2018; 22: 229–239.
- Feng Y-P, Yang T-S, Chung C-H, Chien W-C, Wong C-S. Early childhood general anesthesia exposure associated with later developmental delay: a national population-based cohort study. PLoS One 2020; 15: e0238289.
- Ng YT, Chew FT. A systematic review and meta-analysis of risk factors associated with atopic dermatitis in Asia. World Allergy Organ J 2020; 13: 100477.
- Mortz C, Andersen K, Dellgren C, Barington T, Bindslev-Jensen C. Atopic dermatitis from adolescence to adulthood in the TOACS cohort: prevalence, persistence and comorbidities. Allergy 2015; 70: 836–845.
- Czarnowicki T, He H, Krueger JG, Guttman-Yassky E. Atopic dermatitis endotypes and implications for targeted therapeutics. J Allergy Clin Immunol 2019; 143: 1–11.
- Prescott SL, Macaubas C, Smallacombe T, Holt BJ, Sly PD, Holt PG. Development of allergen-specific T-cell memory in atopic and normal children. The Lancet 1999; 353: 196–200.
- Kawamoto N, Kaneko H, Takemura M, Seishima M, Sakurai S, Fukao T, et al. Age-related changes in intracellular cytokine profiles and Th2 dominance in allergic children. Pediatr Allergy Immunol 2006; 17: 125–133.
- Jafarzadeh A, Hadavi M, Hassanshahi G, Rezaeian M, Vazirinejad R. General anesthetics on immune system cytokines: a narrative review article. Anesth Pain Med 2020; 10: e103033.
- Lee J, Lozano-Ruiz B, Yang FM, Fan DD, Shen L, González-Navajas JM. The multifaceted role of Th1, Th9, and Th17 cells in immune checkpoint inhibition therapy. Front Immunol 2021; 12: 625667.
- Jin Z, Mendu SK, Birnir B. GABA is an effective immunomodulatory molecule. Amino Acids 2013; 45: 87–94.