ORIGINAL REPORT
Prognostic Value of the Neutrophil-to-lymphocyte Ratio, Platelet-to-lymphocyte Ratio and Monocyte-to-lymphocyte Ratio in Melanoma Patients: A Cohort Study
Sümeyre Seda ERTEKIN1,2, Cristina MANGAS1, Constanza RIQUELME-MC LOUGHLIN1, Cristina CARRERA1,3, Josep MALVEHY1,3, Susana PUIG1,3 and Sebastian PODLIPNIK1,3
1Department of Dermatology, Hospital Clinic of Barcelona, University of Barcelona, Barcelona, Spain, 2Department of Dermatology, Koç University School of Medicine, Istanbul, Turkey, 3Institut d’Investigacions Biomediques August Pi I Sunyer (IDIBAPS), Barcelona, Spain
Abstract
The prognostic value of the neutrophil-lymphocyte ratio, platelet-lymphocyte ratio and monocyte-lymphocyte ratio in patients with melanoma has yielded controversial results in the literature. A retrospective single-centre cohort study was conducted from 1998 to 2020, including patients diagnosed with invasive melanoma. A total of 2,721 patients were included in the study. The median follow-up was 8.23 years (IQR 4.41–13.25). The median baseline neutrophil-lymphocyte ratio, platelet-lymphocyte ratio and mono-cyte-lymphocyte ratio values increased significantly (p < 0.001) with the increasing American Joint Committee on Cancer stage. The optimal cut-off values for neutrophil-lymphocyte ratio, platelet-lymphocyte ratio and monocyte-lymphocyte ratio were determined as 2.1, 184 and 0.2, respectively. In the multivariate analysis, high levels of neutrophil-lymphocyte ratio (≥ 2.1), platelet-lymphocyte ratio (≥ 184) and monocyte-lymphocyte ratio (≥ 0.2) were independently associated with significantly shorter melanoma-specific survival (neutrophil-lymphocyte ratio: HR 1.30, 95% CI 1.06–1.60, p = 0.013; platelet-lymphocyte ratio: HR 1.37, 95% CI 1.06–1.76, p = 0.014; monocyte-lymphocyte ratio: HR 1.29, 95% CI 1.05–1.58, p = 0.015) and overall survival (neutrophil-lymphocyte ratio: HR 1.39, 95% CI 1.19–1.64, p < 0.001; platelet-lymphocyte ratio: HR 1.44, 95% CI 1.19–1.74, p < 0.001; monocyte-lymphocyte ratio: HR 1.42, 95% CI 1.21–1.66, p < 0.001). High levels of neutrophil-lymphocyte ratio and monocyte-lymphocyte ratio were also associated with poor relapse-free survival, while platelet-lymphocyte ratio was not. In conclusion, baseline neutrophil-lymphocyte ratio, platelet-lymphocyte ratio and monocyte-lymphocyte ratio were identified as independent predictors for the prognosis of melanoma.
Key words: biomarkers; neutrophil-to-lymphocyte ratio; melanoma; platelet-to-lymphocyte ratio; prognosis; survival.
SIGNIFICANCE
The counts and ratios of peripheral neutrophils, lymphocytes and platelets are readily available and have proven valuable in predicting the prognosis of various malignant tumours. Our study specifically highlights the significance of these markers in primary cutaneous melanoma, where a high baseline neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio and monocyte-to-lymphocyte ratio independently correlate with a shorter lifespan. By identifying high-risk patients through these non-invasive measures, clinicians can guide interventions and treatment options, potentially enhancing patient prognosis and overall quality of life.
Citation: Acta Derm Venereol 2024; 104: adv27571. DOI https://doi.org/10.2340/actadv.v104.27571.
Copyright: © 2024 The Author(s). Published by MJS Publishing, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/).
Submitted: Nov 20, 2023; Accepted: Feb 14, 2024; Published: Apr 24, 2024
Corr: Susana Puig, MD, Melanoma Unit, Dermatology Department, Hospital Clinic Barcelona, Villarroel 170, ES-08036, Barcelona, Spain. E-mail: susipuig@gmail.com
Competing interests and funding: The authors have no conflict of interest to declare.
INTRODUCTION
The incidence of melanoma has been increasing globally over recent decades, and it remains the leading cause of death by skin cancer (1). In the era of available targeted therapies and immunotherapy for melanoma, identifying useful biomarkers is increasingly important as they can improve prognosis and patient outcomes. These biomarkers may include laboratory parameters, molecular patterns or genetic profiles, typically obtained from tumour tissue or peripheral blood samples. Despite extensive research efforts, no ideal prognostic biomarker currently exists in the melanoma field.
In recent years, mounting evidence indicates that cancer-related host immune responses play an essential role in tumour development, disease progression and metastasis in various cancers, including melanoma (2–4). As systemic inflammation alters the composition of circulating peripheral blood cells, some haematological indices can serve as prognostic biomarkers in cancer patients. Changes in the peripheral blood cell counts are best represented by their ratios, namely, the neutrophil-to-lymphocyte ratio (NLR), the platelet-to-lymphocyte ratio (PLR), and the monocyte-to-lymphocyte ratio (MLR). All these ratios have been shown to be valuable biomarkers of prognosis in terms of survival in various types of cancer, as high values usually correlate with worse outcomes (5–7). Moreover, they are easy, fast to perform, affordable and readily available.
Recently, a growing body of research indicates that elevated baseline NLR, PLR and MLR values are associated with decreased melanoma-specific survival (MSS), overall survival (OS) and relapse-free survival (RFS) in patients with early-stage and advanced melanoma (8–12). However, there are also some contradictory results (13–15). Therefore, these haematologic markers’ prognostic significance and optimal cut-off values still need to be fully elucidated.
The aim of this study was to assess the impact of the pre-treatment NLR, PLR and MLR on survival in patients with melanoma and to determine optimal cut-off values to facilitate their use in clinical practice.
MATERIALS AND METHODS
Study design and patients
A retrospective cohort study including patients with invasive melanoma at the Hospital Clinic of Barcelona, Spain, was undertaken between January 1998 and January 2020. Data were obtained from a prospectively collected melanoma database in our institute. This database mainly includes patients of Mediterranean origin living in the Catalonia region. The 8th edition of the American Joint Committee on Cancer (AJCC) melanoma staging system was used for classifying melanomas, and our institute’s standardized protocol was followed in managing the patients (16).
Only patients with biopsy-proven invasive melanoma were included in the study. Patients were excluded if no complete blood count was available at the time of histopathological diagnosis of melanoma. Patients were also excluded if absolute neutrophil counts, platelet counts, monocyte counts or lymphocyte counts were above twice the upper limit or below twice the lower limit of the institutional normal range.
The study was approved by the Clinical Research Ethics Committee of the Hospital Clinic of Barcelona (institutional review board number HCB/2015/0298). All procedures performed in studies involving human participants were in accordance with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines were followed for conducting and reporting this observational study.
Variables and outcomes
Clinicopathological variables included patient age and sex, AJCC stage, tumour site, histopathological subtype, Breslow thickness, ulceration, mitotic index and regression.
The following blood ratios were calculated: NLR, PLR and LMR. These ratios were calculated from absolute blood values as follows: NLR = absolute neutrophil count (number of neutrophils/µl) divided by absolute lymphocyte count (number of neutrophils/µl), PLR = absolute platelet count divided by absolute lymphocyte count, and LMR = absolute monocyte count divided by absolute lymphocyte count. NLR, PLR and LMR were evaluated as independent variables.
The primary outcome measure was melanoma-specific survival (MSS), defined as the time from diagnosis to the date of death from the melanoma or last follow-up. Secondary outcome measures were overall survival (OS), recurrence-free survival (RFS) and distant metastasis-free survival (DMFS). OS was defined as the time from primary melanoma excision to the date of death. RFS was defined as the time from the primary melanoma excision to the date of the first locoregional or distant metastasis. DMFS was defined as the time from primary melanoma excision to the date of the first distant metastasis diagnosis.
Statistical analyses
Categorical variables were presented as frequencies and percentages, and continuous variables were presented as median and interquartile range (IQR). Pearson’s χ2 test was used for categorical variables and trend test for ordinal variables. The Kruskal–Wallis method was used to compare continuous independent variables. The median follow-up of the cohort was estimated using the reverse Kaplan–Meier estimator.
The maximally selected rank statistics from the Maxstat R package (version 2.7.3) was used to determine the optimal cut-off values for NLR, PLR and MLR. This outcome-based statistical method maximizes the log-rank statistics and provides a cut-off value that enables optimal separation between groups regarding the primary outcome of our study (MSS).
To prevent immortal time bias in the setting of multiple primary melanomas, we used worst-case analysis, in which we included only the melanoma with the worst pathological characteristics (highest Breslow thickness and its corresponding ulceration status) for each patient as it was the most likely to be associated with MSS.
Survival probabilities were estimated with the Kaplan–Meier method and compared using the log-rank test. Curves were calculated using the “survfit” function in the “survival” package (version 3.4.0) and plotted with the “survminer” package (version 0.4.9) in R. Univariate and multivariate Cox proportional hazard models were created with the “coxph” function in the “survival” package (version 3.4.0) in R. To adjust for other covariates (age, gender, ulceration and Breslow), we performed the Cox proportional hazards regression using the “coxph” function in R. Hazard ratios (HR) with a 95% confidence interval (CI) were reported. Hazard models were adjusted for main prognostic variables such as age at diagnosis, gender, Breslow index and ulceration. We used independent models for each ratio (NLR, PLR and MLR) in the multivariate analysis to avoid multicollinearity.
All statistical analyses were performed using the R statistical programming language, R version 4.2.2 (2022-10-31) (17). Statistical significance was determined to be p < 0.05, and the p-value was 2-sided.
RESULTS
Patient population
A total of 5,036 melanoma patients were eligible for inclusion. After applying exclusion criteria (no laboratory test available at diagnosis [n = 1,903], in-situ melanoma [n = 380], outlier values for inflammatory markers [n = 30], no follow-up [n = 2]), 2,721 patients were included for analysis. The median follow-up time was 8.23 years (IQR: 4.41–13. 25).
The baseline characteristics of the study population are summarized in Table I. Nearly half of the patients (49.5%) were male, and the median age at diagnosis was 57.0 years (IQR: 43.3–69.8). The most common tumour location was the trunk (44.7%). The most common histopathological subtype was superficial spreading melanoma (64.8%), followed by nodular melanoma (16.0%). The median Breslow index was 1.3 mm (IQR: 0.8–2.8). The positivity rate for further histopathologic characteristics was as follows: ulceration 25.6%, mitosis 55.8% and regression 45.1%. According to AJCC 8th edition, 1,530 patients (57.0%) had stage I melanoma at first diagnosis, 615 (23.0 %) stage II, 499 (18.4%) stage III, and 42 (1.6%) stage IV.
Variation in baseline neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio and monocyte-to-lymphocyte ratio
The median baseline NLR of the whole cohort was 2.1 (IQR: 1.6–2.8), the median PLR was 125.3 (IQR: 98.3–160.7), and the median MLR was 0.2 (IQR: 0.1–0.2) (Table I).
Differences in baseline NLR, PLR and MLR among patient subgroups stratified by disease stage, age and sex are summarized in Fig. 1.
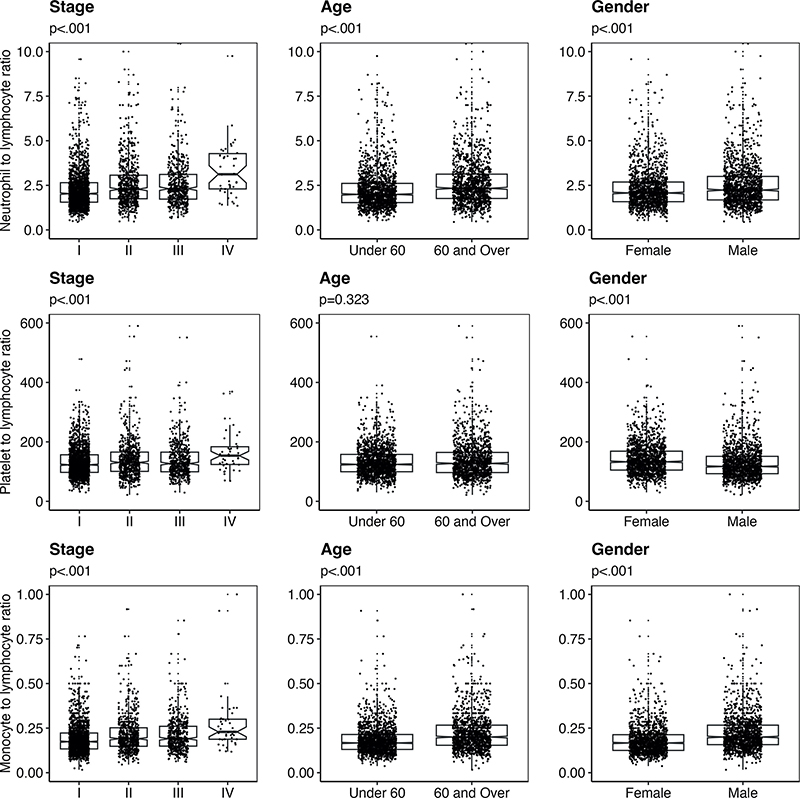
Fig. 1. Variation in baseline neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR) and monocyte-lymphocyte ratio (MLR). Box plots demonstrating baseline NLR, PLR and MLR in patients according to disease stage, age and gender. Each point on the scatter plot represents an individual patient within the subgroup specified.
The median baseline NLR (p < 0.001), PLR (p < 0.001) and MLR (p < 0.001) values increased significantly with the increasing AJCC stage. Patients aged 60 and over had significantly higher baseline NLR (p < 0.001) and MLR (p < 0.001) than patients under 60, but median PLR did not change significantly between older and younger patients. Male patients demonstrated significantly higher baseline NLR (p < 0.001) and MLR (p < 0.001) but significantly lower baseline PLR (p < 0.001) than female patients.
SURVIVAL ANALYSIS
A total of 601 (22.1%) patients relapsed, and 413 patients (15.1%) died from melanoma. The NLR cut-off value for MSS was determined as 2.1, PLR as 184 and MLR as 0.2 with maximally selected rank statistics.
The Kaplan–Meier curves showed that the MSS, OS and RFS rates of the low NLR group (< 2.1) were significantly higher compared with that of the high NLR group (≥ 2.1) (p < 0.001). Kaplan–Meier curves to compare survival between high (< 184) and low PLRs (≥ 184) showed statistically significant differences in MSS (p = 0.002), OS (p < 0.001) and RFS (p = 0.03). Lastly, each of the MSS, OS and RFS rates were significantly decreased among the high MLR (≥ 0.2) group versus the low-MLR (< 0.2) group (p < 0.001) (Fig. 2). Data related to DMFS are shown in Fig. S1.
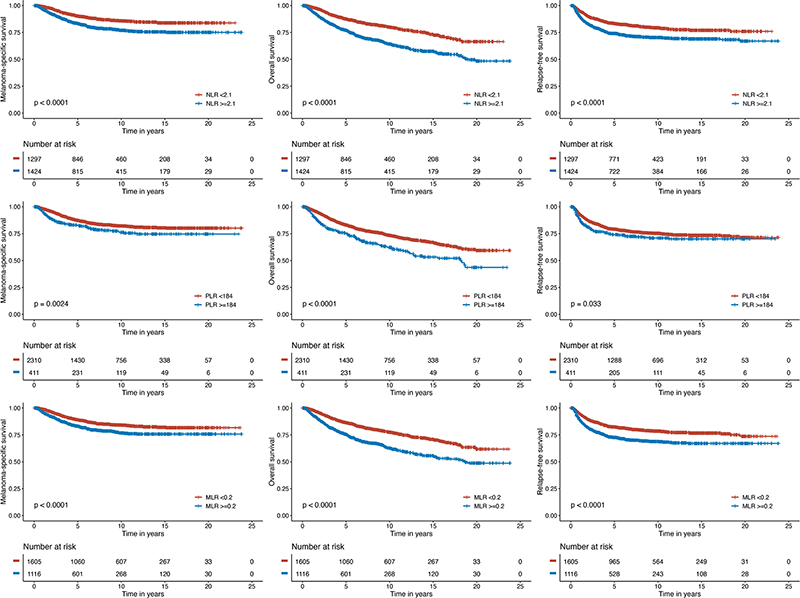
Fig. 2. Melanoma-specific survival (MSS), overall survival (OS) and relapse-free survival (RFS) analysis. Kaplan–Meier survival curves demonstrating survival probability with increasing time after diagnosis in patients with above cut-off neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR) and monocyte-lymphocyte ratio (MLR) (blue) and below cut-off NLR, PLR and MLR (red).
Univariate and multivariate Cox regression models
The relationship of MSS, OS and RFS with histopathological prognostic parameters and inflammatory biomarkers was investigated using univariate and multivariate Cox regression analysis and the results are given in Tables II–IV, respectively.
Univariate analysis revealed the following factors to be significantly associated with MSS: male sex (HR 1.66, 95% CI 1.36–2.02, p ≤ 0.001), age ≥ 60 (HR 1.72, 95% CI 1.41–2.09, p ≤ 0.001), increasing Breslow index (HR 1.07, 95% CI 1.06–1.08, p ≤ 0.001), presence of ulceration (HR 4.94, 95% CI 4.05–6.01, p ≤ 0.001), higher NLR (HR 1.75, 95% CI 1.43–2.14, p ≤ 0.001), higher PLR (HR 1.46, 95% CI 1.14–1.87, p = 0.002), and higher MLR (HR 1.53, 95% CI 1.26–1.86, p = 0.001). Multivariate regression analysis models found that high NLR, high PLR and high MLR were independent predictors of decreased MSS (NLR: HR 1.33, 95% CI 1.08–1.63, p = 0.008; PLR: HR 1.35, 95% CI 1.05–1.75, p = 0.021; MLR: HR 1.28, 95% CI 1.05–1.58, p = 0.017). Gender, age, Breslow index and ulceration also remained independently associated with MSS in our models (Table II).
In the multivariate Cox regression model for OS, each of the high NLR value (≥ 2.1), high PLR value (≥ 184) and high MLR value (≥ 0.2) were significantly associated with a shorter OS after adjusting for age of diagnosis, gender, Breslow thickness and ulceration (NLR: HR 1.39, 95% CI 1.19–1.64, p < 0.001; PLR: HR 1.44, 95% CI 1.19–1.74, p < 0.001; MLR: HR 1.42, 95% CI 1.21–1.66, p < 0.001) (Table III).
In the multivariate Cox regression model for RFS, a high NLR value (≥ 2.1) and a high MLR value (≥ 0.2) were significantly and independently associated with an increased risk of progression. Meanwhile high PLR was not (NLR: HR 1.21, 95% CI 1.02–1.43, p = 0.026; PLR: HR 1.10, 95% CI 0.88–1.37, p = 0.386; MLR: HR 1.32, 95% CI 1.12–1.56, p = 0.001) (Table IV).
Data related to univariate and multivariate Cox regression models for DFMS are shown in Table SI.
DISCUSSION
This retrospective cohort study, including 2,721 patients with invasive melanoma at a national referral centre, analysed the effectiveness of different baseline haematologic inflammatory biomarkers to predict MSS, OS and RFS in cutaneous melanoma patients. Our findings show that NLR, PLR and MLR provide useful prognostic information regarding survival. Each of the high NLR (> 2.1), high PLR (> 184) and high MLR (> 0.2) was independently associated with worse MSS and OS in patients with cutaneous melanoma.
Haematological inflammatory parameters like NLR, PLR and MLR have gained significant attention as readily available and non-invasive prognostic cancer biomarkers in recent years, and an increasing number of studies have confirmed the association of these biomarkers with clinical outcomes in patients with various malignancies. Cancer-associated inflammation builds up a protumorigenic tumour microenvironment and is a crucial determinant of cancer progression and survival (18). For reasons not yet fully elucidated, cancer-associated inflammatory response leads to alterations in peripheral blood cell composition of myeloid and lymphoid lineages, which favours tumour progression, angiogenesis and metastasis (19). Neutrophils, platelets and monocytes from the myeloid lineage are mainly protumorigenic; conversely, NK cells and T-cells from the lymphoid lineage are critical for immune surveillance and show antitumorigenic effects (20, 21). Thus, their relative values in the form of NLR, PLR and MRL capture the balance between the detrimental effects of protumorigenic cells and the beneficial effects of lymphocyte-mediated adaptive immunity, making them potentially valuable prognostic markers.
Many studies have investigated the impact of NLR on survival outcomes in patients with localized and metastatic cutaneous melanoma. In the present study, Breslow thickness, ulceration, male sex and age > 60 were all predictors of decreased survival in the multivariate analyses. Independent of these well-known poor prognostic clinicopathologic variables, an elevated NLR predicted worse overall and melanoma-specific survival. This finding is in line with most of the previous studies, which demonstrated that a high NLR in the initial diagnosis of melanoma was associated with poor MSS and OS (10, 19, 22–25). Different hazard ratios for OS or MSS were reported in the different studies changing from 1.25 to 6.98; these differences are most likely attributable to the variances in the inclusion criteria utilized in each study and the resulting diversity within the study populations (10, 26–28). Interestingly, high HR (> 4) is usually reported in studies that included only stage III or IV metastatic melanomas, raising the question of whether NLR would be a more robust predictive marker of survival for melanoma in late stages than in earlier stages (27, 29, 30). This might be explained by a more pronounced inflammatory response generated by the late-stage melanomas due to a higher tumour burden. Very few studies examined the role of haematological inflammatory indexes on survival in early-stage melanomas, and they found some contradictory results (10, 19, 28). In the present study, by including all melanoma stages from I–IV, we found that a high NLR predicted decreased OS and MSS. Our results align with most of the previous studies that examined similar cohorts, with the exception of the study of Wade et al. (14), in which the authors conversely found that a high NLR was associated with better OS and RFS in stage I–III melanoma patients (14, 22, 23). However, these paradoxical data could not be validated in other studies so far and still need to be further investigated.
One of the most interesting uses of NLR in melanoma patients is predicting the risk of melanoma progression and response to systemic treatments. These studies usually involved stage III and/or IV patients and found that higher baseline NLR values correlated with worse progression-free survival and/or reduced response to immunotherapy or targeted therapy (29, 31–33). Conversely, one study investigated the association between baseline NLR and the risk of recurrence in stage I to III melanoma patients and failed to show any significant association (19). In our present study, a high NLR was significantly associated with worse RFS (HR: 1.21) in a large cohort of patients, including stage I–IV melanomas. This finding suggests that NLR could be useful in identifying patients with localized disease who are at high risk of disease recurrence and thus may benefit from systemic adjuvant therapies and more intensive follow-up protocols.
PLR is another widely investigated inflammatory marker for its prognostic significance in many cancers, including melanoma. Many studies have demonstrated that a high PLR was associated with poor OS in melanoma patients (34, 35). These studies included different patient cohorts with different stages and subtypes of melanomas. They also used different cut-off values for PLR, changing from 99 to 206 (36). In our study, high PLR was a predictor of worse OS and MSS but not PFS in patients with melanoma. Similar to our findings, a recent meta-analysis investigating the prognostic value of the PLR in patients with melanoma found that an elevated PLR was associated with poor OS but not PFS (36). However, another meta-analysis failed to show any obvious association between PRL and OS or PFS in patients with melanoma (37). Small sample sizes in these studies were a significant limitation, and further prospective studies are needed to clarify contradictory results.
The prognostic role of the LMR (lymphocyte to monocyte ratio) or MLR has been investigated less in the field of melanoma, and the results were contradictory. In some of the studies, lower LMR was associated with an increased mortality risk in patients with melanoma; however, this association could not be shown in others (22, 38, 39). In the present study, higher MLR was associated with poor OS, MSS and RFS. To our knowledge, our study was the first to explore the prognostic value of MLR on RFS and adds valuable information to the current literature.
Limitations
The limitations of this study are its single-centre and retrospective design. Moreover, the potential confounding factors and comorbidities affecting the haematological inflammatory indexes had to be ignored, as there was no specific information in the medical reports. However, the limitations inherent in this study are outweighed by its numerous strengths. First, to the best of our knowledge, our study has the largest sample size among published studies examining the prognostic significance of haematologic inflammatory indexes in melanoma. Second, we have included all clinical stages of melanoma, and there was a balanced proportion between stages. Third, we used MSS as the primary endpoint instead of OS because an elevated NLR is associated with increased all-cause mortality in the general population (40). Lastly, the cut-off values for NLR, PLR and MLR in our study were determined by using maximally selected rank statistics, which is believed to be a more appropriate method than ROC analysis, which was used in the majority of previous studies (33).
Conclusion
In our study, baseline NLR, PLR and MLR were identified as independent predictors for the prognosis of melanoma in a large cohort of patients. These biomarkers could potentially be useful in routine clinical practice to identify patients who may benefit from enhanced surveillance. Moreover, incorporating these haematological markers into the machine learning algorithms and nomograms of melanoma holds the promise of a better prognosis and thus, more individualized and comprehensive care for patients. Nevertheless, further multicentre prospective studies are necessary to validate our findings and standardization is warranted before implementing it in routine clinical practice.
ACKNOWLEDGEMENTS
Funding sources: The research at the Melanoma Unit in Hospital Clinic Barcelona is partially financed by grants PI15/00716, PI15/00956, PI18/00419 and PI22/01457, from Fondo de Investigaciones Sanitarias (Spain); and by the CIBER de Enfermedades Raras of the Instituto de Salud Carlos III, Spain. Co-funded by ISCIII-Subdireccion General de Evaluacion and European Regional Development Fund (ERDF), “a way to make Europe”; AGAUR 2017_SGR_1134 and CERCA Programme by Generalitat de Catalunya, Spain; European Commission under the 6th Framework Programme, Contract No. LSHC-CT-2006-018702 (GenoMEL), by the European Commission under the 7th Framework Programme, Diagnoptics, and the European Commision under the HORIZON2020 Framework Programme, iTobos, Qualitop, MELCAYA; the National Cancer Institute (NCI) of the US National Institute of Health (NIH) (CA83115); a grant from “Fundació La Marató de TV3” 201331-30, Catalonia, Spain; and a grant from “Fundación Científica de la Asociación Española Contra el Cáncer” GCB15152978SOEN, Spain. Part of the work was carried out at the Esther Koplowitz Center, Barcelona.
IRB approval status: The research protocol received approval from the Research Ethics Committee of the Hospital Clinic of Barcelona (Institutional Review Board number HCB/2015/0298).
REFERENCES
- Arnold M, Singh D, Laversanne M, Vignat J, Vaccarella S, Meheus F, et al. Global burden of cutaneous melanoma in 2020 and projections to 2040. JAMA Dermatol 2022; 158: 495–503.
- Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol 2014; 15: e493–503.
- Coussens LM, Werb Z. Inflammation and cancer. Nature 2002; 420: 860–867.
- Liu J, Lin PC, Zhou BP. Inflammation fuels tumor progress and metastasis. Curr Pharm Des 2015; 21: 3032–3040.
- Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, Ocaña A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 2014; 106: dju124.
- Howard R, Kanetsky PA, Egan KM. Exploring the prognostic value of the neutrophil-to-lymphocyte ratio in cancer. Sci Rep 2019; 9: 19673.
- Zhou X, Du Y, Huang Z, Xu J, Qiu T, Wang J, et al. Prognostic value of PLR in various cancers: a meta-analysis. PLoS One 2014; 9: e101119.
- Capone M, Giannarelli D, Mallardo D, Madonna G, Festino L, Grimaldi AM, et al. Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J Immunother Cancer 2018; 6: 74.
- Ferrucci PF, Ascierto PA, Pigozzo J, Del Vecchio M, Maio M, Antonini Cappellini GC, et al. Baseline neutrophils and derived neutrophil-to-lymphocyte ratio: prognostic relevance in metastatic melanoma patients receiving ipilimumab. Ann Oncol 2016; 27: 732–738.
- Lino-Silva LS, Salcedo-Hernández RA, García-Pérez L, Meneses-García A, Zepeda-Najar C. Basal neutrophil-to-lymphocyte ratio is associated with overall survival in melanoma. Melanoma Res 2017; 27: 140–144.
- Zhan H, Ma JY, Jian QC. Prognostic significance of pretreatment neutrophil-to-lymphocyte ratio in melanoma patients: a meta-analysis. Clin Chim Acta 2018; 484: 136–140.
- Failing JJ, Yan Y, Porrata LF, Markovic SN. Lymphocyte-to-monocyte ratio is associated with survival in pembrolizumab-treated metastatic melanoma patients. Melanoma Res 2017; 27: 596–600.
- Minowa T, Kato J, Hida T, Horimoto K, Sato S, Sawada M, et al. Prognostic role of platelet to lymphocyte and lymphocyte to monocyte ratios in advanced melanoma treated with anti-programmed death–1. Eur J Dermatol 2018; 28: 705–707.
- Wade RG, Robinson AV, Lo MCI, Keeble C, Marples M, Dewar DJ, et al. Baseline neutrophil-lymphocyte and platelet-lymphocyte ratios as biomarkers of survival in cutaneous melanoma: a multicenter cohort study. Ann Surg Oncol 2018; 25: 3341–3349.
- Cohen JT, Miner TJ, Vezeridis MP. Is the neutrophil-to-lymphocyte ratio a useful prognostic indicator in melanoma patients? Melanoma Manag 2020; 7: Mmt47.
- Podlipnik S, Carrera C, Sánchez M, Arguis P, Olondo ML, Vilana R, et al. Performance of diagnostic tests in an intensive follow-up protocol for patients with American Joint Committee on Cancer (AJCC) stage IIB, IIC, and III localized primary melanoma: a prospective cohort study. J Am Acad Dermatol 2016; 75: 516–524.
- Team RC. R: A Language and Environment for Statistical Computing. Vienna, 2023.
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature 2008; 454: 436–444.
- Robinson AV, Keeble C, Lo MCI, Thornton O, Peach H, Moncrieff MDS, et al. The neutrophil-lymphocyte ratio and locoregional melanoma: a multicentre cohort study. Cancer Immunol Immunother 2020; 69: 559–568.
- Shaul ME, Fridlender ZG. Neutrophils as active regulators of the immune system in the tumor microenvironment. J Leukoc Biol 2017; 102: 343–349.
- Gonzalez H, Hagerling C, Werb Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev 2018; 32: 1267–1284.
- Fortes C, Mastroeni S, Zappalà AR, Passarelli F, Ricci F, Abeni D, et al. Early inflammatory biomarkers and melanoma survival. Int J Dermatol 2023; 62: 752–758.
- Pinto-Paz ME, Cotrina-Concha JM, Benites-Zapata VA. Mortality in cutaneous malignant melanoma and its association with neutrophil-to-lymphocyte ratio. Cancer Treat Res Commun 2021; 29: 100464.
- Wagner NB, Luttermann F, Gassenmaier M, Forschner A, Leiter U, Garbe C, et al. Absolute and relative differential blood count predicts survival of AJCC stage I–II melanoma patients scheduled for sentinel lymph node biopsy. Australas J Dermatol 2020; 61: e310–e318.
- Davis JL, Langan RC, Panageas KS, Zheng J, Postow MA, Brady MS, et al. Elevated blood neutrophil-to-lymphocyte ratio: a readily available biomarker associated with death due to disease in high risk nonmetastatic melanoma. Ann Surg Oncol 2017; 24: 1989–1996.
- Rosner S, Kwong E, Shoushtari AN, Friedman CF, Betof AS, Brady MS, et al. Peripheral blood clinical laboratory variables associated with outcomes following combination nivolumab and ipilimumab immunotherapy in melanoma. Cancer Med 2018; 7: 690–697.
- Cananzi FC, Dalgleish A, Mudan S. Surgical management of intraabdominal metastases from melanoma: role of the neutrophil to lymphocyte ratio as a potential prognostic factor. World J Surg 2014; 38: 1542–1550.
- Ding Y, Zhang S, Qiao J. Prognostic value of neutrophil-to-lymphocyte ratio in melanoma: evidence from a PRISMA-compliant meta-analysis. Medicine (Baltimore) 2018; 97: e11446.
- Ferrucci PF, Gandini S, Battaglia A, Alfieri S, Di Giacomo AM, Giannarelli D, et al. Baseline neutrophil-to-lymphocyte ratio is associated with outcome of ipilimumab-treated metastatic melanoma patients. Br J Cancer 2015; 112: 1904–1910.
- Li Y, Meng Y, Sun H, Ye L, Zeng F, Chen X, et al. The prognostic significance of baseline neutrophil-to-lymphocyte ratio in melanoma patients receiving immunotherapy. J Immunother 2022; 45: 43–50.
- Cassidy MR, Wolchok RE, Zheng J, Panageas KS, Wolchok JD, Coit D, et al. Neutrophil to lymphocyte ratio is associated with outcome during ipilimumab treatment. EBioMedicine 2017; 18: 56–61.
- Finon A, Zaragoza J, Maillard H, Beneton N, Bens G, Samimi M, et al. A high neutrophil to lymphocyte ratio prior to BRAF inhibitor treatment is a predictor of poor progression-free survival in patients with metastatic melanoma. Eur J Dermatol 2018; 28: 38–43.
- Ma J, Kuzman J, Ray A, Lawson BO, Khong B, Xuan S, et al. Neutrophil-to-lymphocyte ratio (NLR) as a predictor for recurrence in patients with stage III melanoma. Sci Rep 2018; 8: 4044.
- Wang E, Huang H, Tang L, Tian L, Yang L, Wang S, et al. Prognostic significance of platelet lymphocyte ratio in patients with melanoma: a meta-analysis. Medicine (Baltimore) 2021; 100: e27223.
- Qi Y, Zhang Y, Fu X, Wang A, Yang Y, Shang Y, et al. Platelet-to-lymphocyte ratio in peripheral blood: a novel independent prognostic factor in patients with melanoma. Int Immunopharmacol 2018; 56: 143–147.
- Zhang F, Gong W. Prognostic value of the platelet-to-lympho-cyte ratio in patients with melanoma: a meta-analysis. Front Oncol 2020; 10: 1116.
- Han SN, Feng SJ, Liu Y. Prognostic and clinicopathological significance of the platelet-to-lymphocyte ratio in melanoma: a meta-analysis involving 2099 patients. Kaohsiung J Med Sci 2021; 37: 55–62.
- Gandini S, Ferrucci PF, Botteri E, Tosti G, Barberis M, Pala L, et al. Prognostic significance of hematological profiles in melanoma patients. Int J Cancer 2016; 139: 1618–1625.
- Iacono D, Basile D, Gerratana L, Vitale MG, Pelizzari G, Cinausero M, et al. Prognostic role of disease extent and lymphocyte–monocyte ratio in advanced melanoma. Melanoma Res 2019; 29: 510–515.
- Chen Y, Wang W, Zeng L, Mi K, Li N, Shi J, et al. Association between neutrophil-lymphocyte ratio and all-cause mortality and cause-specific mortality in US adults, 1999–2014. Int J Gen Med 2021; 14: 10203–10211.