Chronic pruritus is a common symptom, associated with several severe medical conditions, great psychological burden, and reduced quality of life. It also poses socio-economic challenges concerning patients’ work loss and healthcare costs. In Germany, medical rehabilitation programmes represent an integral part of the medical care of patients with chronic inflammatory skin diseases. However, such programmes play only a rudimentary role in the treatment of other dermatological diseases, such as dermatological oncology, genetic skin diseases, and chronic pruritus. Therefore, a specific antipruritic dermatological rehabilitation programme was developed in cooperation between the Department of Dermatology of the Medical Rehabilitation Center Bad Bentheim and the Center for Chronic Pruritus of the University Hospital of Muenster, Germany. This prospective study compared short-term patient-reported outcomes (n = 121) at the beginning and end of the rehabilitation programme. The majority of subjects had chronic pruritus on primary diseased, inflamed skin. Significant improvements in pruritus intensity (p ≤ 0.001), quality of life (p ≤ 0.001), anxiety symptoms (p ≤ 0.001) and depression (p ≤ 0.001), as well as an overall patient-relevant benefit (Patient Benefit Index 2.6 ± 1.06) and treatment-related patients’ satisfaction, were shown. This suggests that implementation of this standardized rehabilitation programme for treatment of patients with chronic pruritus was successful.
Key words: itch; chronic pruritus; rehabilitation; patient-reported outcome; guideline; clinical trials; clinical research.
Accepted Nov 1, 2022; Epub ahead of print Nov 1, 2022
Acta Derm Venereol 2022; 102: adv00831.
DOI: 10.2340/actadv.v102.2930
Corr: Athanasios Tsianakas, Fachklinik Bad Bentheim, Department of Dermatology and Allergology, Am Bade 1, DE-48455 Bad Bentheim, Germany. E-mail a.tsianakas@fk-bentheim.de
SIGNIFICANCE
Chronic pruritus, defined as itch lasting for at least 6 weeks or longer, is a complex medical condition that requires a differentiated and individualized strategy concerning diagnostic and therapeutic options. This study shows the value of a specialized antipruritic rehabilitation programme in the management of patients with chronic pruritus and its potential impact on pruritus intensity, quality of life, anxiety and depression, as well as the overall benefit to patients of a holistic, continuous treatment over 3 weeks, associated with significant improvements in chronic pruritus and its associated consequences.
INTRODUCTION
Chronic pruritus (CP) is defined as itch lasting for at least 6 weeks or longer (1). The point prevalence of CP is estimated as 16.8% among German employees (2) and as 13.5% among the general German population (3). The incidence rate has been estimated at 7% within a period of 12 months (4).
In Germany, more than 20% of individuals have experienced CP during their lifetime (3).
The International Forum for the Study of Itch (IFSI) classifies CP into 3 groups (1):
- Group I: CP on primary diseased, inflamed skin.
- Group II: CP on primary non-diseased, non-inflamed skin.
- Group III: CP with secondary scratch lesions (predominance of secondary scratch lesions, e.g. prurigo nodularis or lichen simplex) and impossibility to be allocated to group I or II).
The origin of CP ranges from classical dermatological inflammatory diseases, such as atopic dermatitis, psoriasis vulgaris or autoimmune bullous diseases (e.g. bullous pemphigoid) via internal medical diseases, such as chronic renal failure or liver diseases, to neurological and psychiatric diseases, such as neuropathy, depression or psychosis. These various origins can be summed up into 6 categories: dermatological, systemic, neurological, psychological, mixed (for patients with more than 1 underlying disease), and other (for unknown origin). Therefore, the task force for CP of the German Dermatological Society recommends an extensive diagnostic programme to investigate the origin of pruritus (5).
Regarding therapy for CP, the detection of its origin is of great importance; however, in up to 45% of patients, no underlying disease or condition can be found (6). By successfully treating the underlying disease, CP might fully disappear or at least improve in many cases. Still, the symptom CP itself may become a disease, often correlating with a long duration of CP, and should be treated with specific antipruritic therapies.
CP is known to have significant impact on health-related quality of life (HRQoL) in affected patients (7) and poses a similar burden to that of chronic pain (8). Furthermore, CP has a significant impact on emotional well-being (9), as it can lead to sleep disorders (10) or even psychiatric diseases (11–13). Hence, there is a strong correlation between psychiatric or psychosomatic diseases, such as depression, anxiety, delusional disorders, and eating disorders and CP (14–16). They may induce and maintain CP, but CP may also lead to higher anxiety and depression scores (4).
Therefore, successful therapy for CP should ideally be organized by medical centres with a specialization in the management of CP.
Since CP can lead to an immense psychological burden, results in social withdrawal, and strongly impacts on efficacy at work, there is great need for an effective long-term management and a specific antipruritic dermatological rehabilitation programme. Therefore, such a programme was developed in close cooperation between the Department of Dermatology of the Medical Center Bad Bentheim (Fachklinik Bad Bentheim, Klinik für Dermatologie und Allergologie) and the Competence Center Chronic Pruritus (KCP) of the University Hospital of Muenster, Germany. This programme extends over a period of 3–4 weeks. During the programme the medical antipruritic therapy according to the European S2k Guideline on CP is initiated, continued, or even intensified. In addition, patients are consulted by a specified psychologist with extensive experience in CP. Treatment includes balneophototherapy containing sulphuric mineral water baths followed by ultraviolet (UV) therapy, relaxation interventions, a dietary consultation, and an individual body workout programme containing physiotherapy, ergotherapy, aquatic therapy and others. For employees, the rehabilitation stay is financed by pension insurance, whereas, for pensioners, the finance is covered by the statutory health insurance.
The very positive feedback of participating patients, who have CP, led to the implementation of this pilot study, which included 121 rehabilitation patients with CP. The aim of the evaluation study was to measure the efficacy of this specific dermatological antipruritic rehabilitation programme on pruritus, but also on quality of life and psychological burden, such as anxiety and depression.
METHODS
Study design
This investigator-initiated, prospective trial evaluating the efficacy of a rehabilitation programme in patients with CP was conducted at the Fachklinik Bad Bentheim, a German dermatological clinic specialized in the acute in-patient treatment and rehabilitation of patients with CP, inflammatory dermatoses and dermato-oncological illnesses. The study was approved by the respective ethics committee (Ärztekammer Niedersachsen, Lower Saxony, Germany) and registered at the German Clinical Trials Register (DRKS00016492). Key inclusion criteria were CP, defined as pruritus lasting 6 weeks or longer, a commitment to a 3-week rehabilitation programme at our clinic and age ≥ 18 years.
Fig. 1 provides an overview of the therapeutic and diagnostic options of the rehabilitation programme, which were applied according to the underlying cause of CP and individual patients’ needs. After giving informed oral and written consent, all patients answered a set of paper-based patient-reported assessments at baseline, i.e. the first day of their hospitalization. The second survey was conducted on the last day of the rehabilitation programme. The rehabilitation was commenced with a thorough examination with regards to diagnosis, co-morbidities as well as psychological burden. During the programme, patients with CP received an intensive topical therapy with topical glucocorticoids, liquor carbonis detergens 5% ointment and daily topical basic therapy, as well as a skin-type adjusted balneophototherapy with narrowband UVB (311 nm) or, in some cases, UVA1 and either thermal salt-water or sulphuric mineral water from the clinic’s wells. Systemic therapy according to the German guidelines for CP were administered when medically indicated (17). This included, as basic treatment, antihistamines up to 4 doses per day (high dose). If not sufficient, systemic treatment with gabapentinoids (gabapentin, pregabalin), antidepressants (such as mirtazapine or paroxetine) or, in therapy-resistant cases, intravenous therapy with naloxone (after exclusion of potential contraindications, such as intake of opioids or significant cardiac arrhythmias) was initiated. If there was a specific dermatosis, disease-specific systemic treatment options were also started if indicated (such as methotrexate, biologics, etc.). During the doctors’ rounds, but also in special training courses, patients received individual training and education concerning their skin disease diagnosis, treatment and general skin care, as well as coping mechanisms. Furthermore, psychosomatic therapy with counselling sessions and different courses for relaxation techniques were applied, and physical treatment, such as physiotherapy or occupational therapy (ergotherapy), was performed (for further details see Fig. 1).
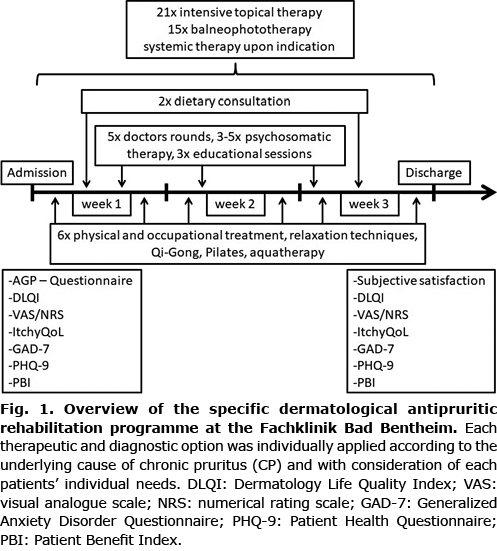
Assessment instruments
To obtain the complex medical history of patients with CP in a standardized manner, the AGP-Questionnaire was used once at the beginning of treatment, assessing course, intensity (measured with a visual analogue scale (VAS), ranging from 0 to 10) and quality of pruritus as well as general health condition and sociodemographic data (7, 18).
To quantify treatment success regarding pruritus intensity, QoL, depression and anxiety, tools were assessed at the beginning of rehabilitation and after completing treatment:
To measure the intensity of pruritus, a VAS as well as a numerical rating scale (NRS, ranging from 0 to 10) were used (19). Mean pruritus, as well as the strongest pruritus, was indicated for the past 24 h before admission and discharge, respectively. In addition, mean itch intensity at admission, as well as the highest and lowest intensity during 4 weeks before admission, was captured.
QoL was measured with the Dermatology Life Quality Index (DLQI), which evaluates the impact of the skin disease and its treatment on the psychological condition of patients, their daily activities, and their social as well as professional participation. The DLQI sum score indicates overall QoL, from no impairment (sum score 0) to greatest impairment (sum score 30) (20).
The GerItchyQoL (German version of the ItchyQoL) measures the subjective reduction in QoL for patients with CP, ranging from 0 to 110 (21, 22). To evaluate the psychological impact, the Generalized Anxiety Disorder Questionnaire (GAD-7), and the Patient Health Questionnaire (PHQ-9) to measure depression were chosen (23, 24). Furthermore, a benefit analysis was performed with the Patient Benefit Index version for pruritus (PBI-P) (25, 26, 44, 45).
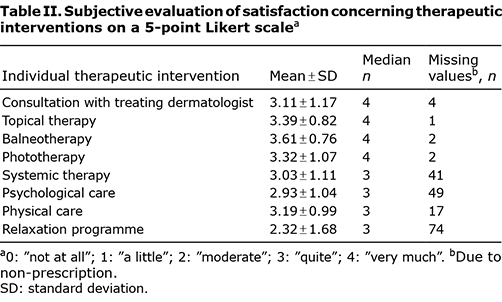
To evaluate different therapeutic options, a questionnaire was developed measuring the subjective satisfaction of patients on a 5-point Likert scale (“not at all”, “a little”, “moderate”, “quite”, “very much”). This questionnaire consists of 8 questions on overall satisfaction as well as satisfaction with doctor-patient interaction, topical, systemic, physical, balneophototherapy and phototherapy and psychological care.
Statistical methods
Statistical analysis was performed with R, a software environment for statistical computing and graphics (27). Patients’ characteristics, as well as patient-reported outcomes, were analysed using descriptive statistics indicating mean, standard deviation and median. Subgroups were formed based on the origin of the pruritus. Dependent t-tests with paired samples were used to compare change in intensity of pruritus, QoL and severity of psychological burden. The PBI-P was calculated by multiplying the goal attainment items with the goal importance items (divided by the sum of all goal importance items) and summing the resulting products, leading to values between 0 (no benefit) and 4 (greatest benefit). The minimum relevant benefit is defined as a global PBI-P score ≥ 1 (25). Graphical presentation was arranged using Microsoft Excel 2010. Statistical significance was assumed at p < 0.05. Due to the exploratory character of the study, no adjustment for multiple testing was performed.
RESULTS
Patient characteristics
Demographics and the distribution of underlying diseases in CP are shown in Table I. In total, 121 patients participated in the study. The most common origin of CP was atopic dermatitis (AD), followed by psoriasis vulgaris (PSO) and chronic nodular prurigo (CNPG), the latter with underlying causes, such as AD, chronic kidney disease, type 2 diabetes mellitus and PSO. Less frequent causes were lichen planus, chronic spontaneous urticaria and Duhring’s disease, or neurological diseases, such as brachioradial CP or notalgia paraesthetica.
Duration, intensity, quality and trigger factors of chronic pruritus
Almost half of the patients had had CP for more than 10 years (47.3%, n = 53). 41.1% (n = 46) were affected for 1–10 years, and only 11.6% for < 1 year (7.1% between 6 and 12 months, 4.5% between 6 weeks and 6 months).
Prior to the rehabilitation treatment, 93 patients (76.9%) had been treated with a topical anti-pruritic therapy and 32.2% (n = 39) had previously received systemic treatment (anti-pruritic and/or anti-inflammatory). Only a minority of the included patients initiated a new anti-inflammatory systemic treatment (2 patients with AD started biologics, 3 patients with PSO started systemic treatment: 1× fumaric acid esters, 1× methotrexate, 1× biologics).
The mean intensity of itch at admission, measured with the VAS, was 5.7 (± 2.3, median 6). The highest and lowest mean intensity during 4 weeks before admission were 7.4 (± 2.1, median 8) and 3.4 cm (± 2.1, median 3). Both mean and highest intensity of itch improved significantly during the rehabilitation treatment, when comparing the past 24 h before admission and discharge (Fig. 2). The mean intensity of pruritus was reduced from moderate pruritus to mild pruritus (28).
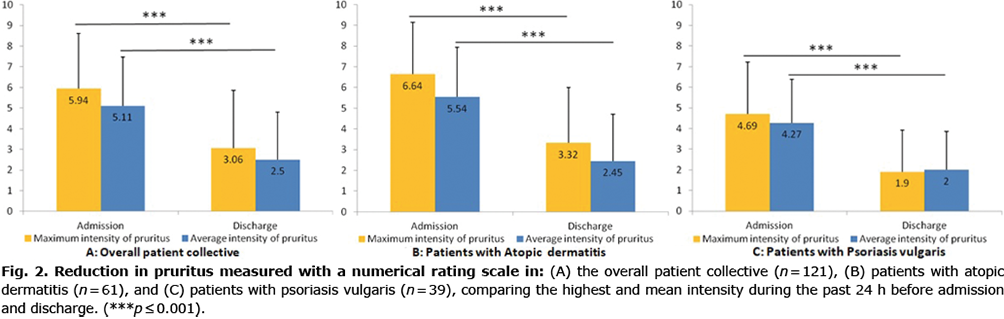
Subgroup analysis showed similar results for patients with AD (n = 61) and PSO (n = 39) for pruritus 24 h before admission and 24 h before discharge. The maximum intensity of pruritus was also reduced significantly (Fig. 2).
The analysis of trigger factors (AGP-Questionnaire) showed a correlation between pruritus and certain activities. Most patients indicated multiple trigger factors for CP (n = 86, 71.1%), whereas 27.3% (n = 33) indicated only 1 trigger factor. Trigger factors were mainly psychological, but external triggers also played a role: emotional (52.9%) and inner tension (43.8%), rest (46.3%), but also sweating (54.6%), warmth of bed (47.1%), and physical exertion (35.5%).
Quality of life at admission and discharge
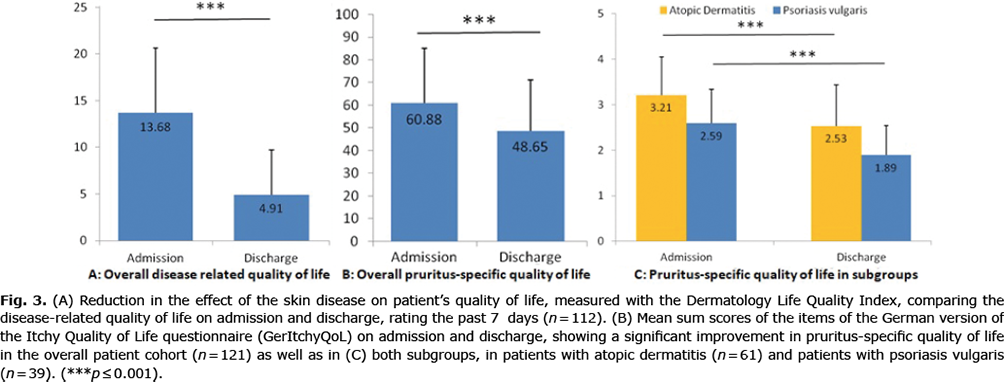
The most common questionnaire measuring QoL in dermatological patients is the DLQI. The DLQI was completed by 112 patients. Upon admission, the DLQI sum score was 13.7 ± 6.9, showing a large effect on patient’s life (29, 30). At discharge, the sum score was reduced to 4.9 ± 4.8, showing a significant improvement (Fig. 3).
Most patients were “sometimes” (n = 33, 27.3%) or “rather often” (n = 37, 30.6%) affected in their QoL. Most patients (74.4%, n = 90) had itching sensations at least once a day. Only 12.4% (n = 15) were affected only multiple times per week and 7 patients (5.8%) only multiple times per month.
In 95 patients (78.5%), the nature of the pruritus was transient, in contrast to only 16 patients (13.2%) indicating continuous pruritus. In most patients, CP was present both during the day and at night (n = 65, 53.8). Twenty-one patients (17.4%) indicated pruritus only during the day and 18 patients (14.9%) indicated solely nocturnal itch.
Pruritus-specific QoL, measured by the GerItchyQol before and after rehabilitation treatment, also showed a significant improvement. With a mean sum score of 60.9 ± 24.1 before treatment, the negative effect of CP could be categorized as “moderate”. The mean sum score improved to 48.7 ± 22.5 at discharge, which is a “mild” impairment in QoL (31) (Fig. 3).
Subgroup analysis in patients with AD and PSO also showed a significant improvement (Fig. 3).
Anxiety and depression at baseline and follow-up
Anxiety, measured by the GAD-7 questionnaire (a validated questionnaire containing 7 questions), decreased significantly on average during the rehabilitation from mild anxiety symptoms (7.9 ± 5.1), which require further monitoring, to minimal anxiety symptoms (4.9 ± 4. 8), requiring no further monitoring or treatment (23). This applied both to patients with PSO and to those with AD (Fig. 4).
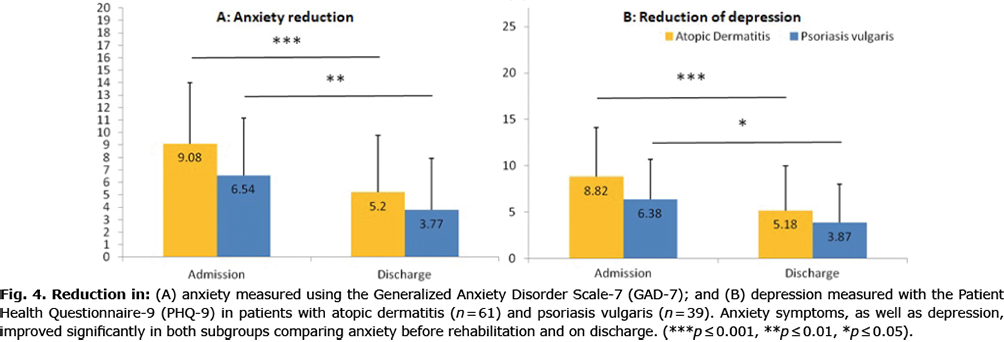
PHQ-9 is a validated questionnaire containing 9 questions asking for signs of depression. It showed a significant reduction in depression and accompanying symptoms, such as loss of interest, sleeping disorders, lack of concentration or loss of appetite. Both in patients with AD and in those with PSO, a significant improvement was found, with mean severity of depression in patients with PSO decreasing from mild (6.4 ± 4.3) to minimal (3.9 ± 4.2) depression, whereas patients with AD remained in the category of mild depression (5.2 ± 4.9) but with a significant improvement (8.8 ± 5.3) (24) (Fig. 4).
Patient-relevant benefit
The PBI-P is a validated questionnaire measuring whether the personal patients’ treatment aims have been reached. PBI-P data were collected for 109 patients on admission and discharge. At baseline the most relevant therapeutic goals (classified as “very important”) were “to no longer experience itching” (93.4%), “to have confidence in the therapy” (90.9%) and “to be healed of all skin alterations” (89.3%). At follow-up (week 3) the items with the most relevant benefit were “to have confidence in the therapy”, “to be free of pain” and “to find a clear diagnosis and therapy”. The least important goals were “to have no fear that the disease will progress”, “to have lower out-of-pocket treatment costs” and “to need less time for daily treatment”.
The mean PBI-P global score was 2.6 ± 1.1, indicating overall patient-relevant benefit from the rehabilitation programme. The vast majority of patients (91.7% of patients who completed both PBI-P questionnaires, the questionnaire on admission as well as on discharge) achieved the score for patient-relevant benefit in PBI-P global score at discharge (PBI-P≥1) (25).
Treatment satisfaction questionnaire
The evaluation of individual therapeutic interventions and their contribution to recovery was completed by 105 patients: 16 patients did not complete the questionnaire. For the majority of the therapeutic options, most patients indicated high satisfaction (see Table II). 43.8% (n = 53) of patients were very satisfied and 19.8% (n = 24) were quite satisfied with the regular consultations with their treating dermatologist during rehabilitation. Also, topical therapy (47.1%, n = 57), balneophototherapy (61.2%, n = 74), phototherapy (51.2%, n = 62) as well as systemic therapy (23.1%, n = 28) showed high satisfaction scores. Overall, most patients benefited “very much” (60.3%) and “quite” (19.1%) from rehabilitation. No patient indicated that he or she was “not at all” satisfied.
DISCUSSION
The aim of this prospective evaluation study was to explore the short-term significance and efficacy of a specific dermatological rehabilitation programme in treating CP. Regarding pruritus intensity, impairment of QoL as well as psychological impairment, participating patients benefited from the rehabilitation stay in many respects. Significant improvement in the mean and highest intensity of pruritus, leading to a reduction in moderate mean pruritus intensity to a mild pruritus intensity, as well as improvement in QoL, show the significance of a structured rehabilitation programme for patients with CP. These findings were supported by the patient-reported benefit as well as high satisfaction with the applied treatment regime.
Concerning patients’ characteristics, such as sex and age, the current patient collective coincides with those of previous studies (17, 32, 38). The results confirm that CP may occur in patients independently of age and is associated with various co-morbidities. In our sample, most frequent causes of CP in decreasing order of frequency were AD, PSO and CNPG. These are chronic diseases and stand for a “typical patient” applying for a dermatological rehabilitation programme in Germany. Criteria for approval of such a rehabilitation include long-standing chronic, therapy-resistant skin diseases with negative impact on QoL, profession and social life (39, 40). In real life, AD and PSO, both chronic inflammatory skin diseases, represent the 2 most common referral diagnoses to dermatological rehabilitation programmes (41). Due to the small subgroup sample sizes in the current study, only subgroup analyses for patients with AD and PSO allowed statistically reliable evaluations. In particular, patients with pruritus-underlying internal diseases, such as diabetes, renal failure or liver diseases, are less likely be referred to our rehabilitation programme, since their underlying diseases are not mainly dermatological. And, especially, CP on primary non-diseased, non-inflamed skin is often diagnosed as CP with a delay of many years, so that adequate treatment is started late. Concerning patients’ ages, which ranged from 38 to 64 years, the current sample represents a patient collective, which profits most from rehabilitation programmes with regards to restoration of working ability.
While it is widely acknowledged that AD and CNPG are accompanied by intense pruritus as their main symptom, PSO as an underlying disease for CP is often overlooked in everyday clinical practice, even though more than 80% of patients with PSO experience pruritus (33, 46). Hence, it is not surprising that, in our sample, PSO was the second most common cause of CP. Concerning pre-treatment with antipruritic substances, the current patient collective received less topical (77%) or systemic (32%) therapies prior to rehabilitation than participants of the validation study of the German itch questionnaire (18). This need for rehabilitation may thus be at least partly due to chronic under-treatment. An explanation for that under-treatment of the current study population might be that these patients are from all over Germany, including both rural and urban areas, whereas the patients from the validation study are largely from more urban areas, where access to dermatological treatment might be easier.
CP is a burdensome disease that can be difficult to treat. In the current sample, most patients had had CP for at least 1 year and for up to more than 10 years, indicating the long journey to successful treatment. Similar to previous studies, QoL was severely reduced, due to the frequency, intensity and nature of the pruritus (5). Most patients received at least 1 anti-pruritic topical therapy or even a systemic therapy prior to rehabilitation, which was perceived as insufficient. Finding the optimal individual therapeutic plan for every patient often remains a challenge for practitioners. In particular, in an outpatient setting, due to a lack of time and resources, it is difficult to meet the needs of patients with CP, as they often experience somatic und psychological comorbidities. In these complex cases, patients can benefit from an interdisciplinary approach, which is more likely to be realized in the context of an in-patient rehabilitation with the possibility of a holistic approach. By composing a complex treatment plan according to comorbidities and adjusting the plan contemporarily in case of adverse reactions or ineffectiveness, a patient-relevant benefit can be achieved, as shown by the analysis of the patient benefit questionnaire. Compared with non-rehabilitation inpatient stays at acute care clinics, rehabilitation programmes offer disease-specific educational sessions, supporting patients’ comprehension of pathogenesis of underlying diseases, leading to a better understanding of trigger factors and their prevention. The great improvement in pruritus may also be explained by the continuous application of therapies, especially topical treatment, as well as UV and balneophototherapy. A mandatory part of the specific antipruritic rehabilitation programme is an individual psychological care covered by psychologists or psychotherapists. Many patients emphasized that that intense psychological intervention had never been offered to them previously, and that it helped them a lot to cope with the disease itself and its negative impact on various aspects of life. In general, the continuous medical and psychological treatment over a period of 3 weeks often leads to a complete healing of skin lesions. This may not be achieved by an intermittent application of therapies, leading to insufficient and short-lasting improvement in pruritus. During the course of 3 weeks of continuous medical surveillance, therapies may be adjusted contemporarily, skipping long waiting times for follow-up appointments. Furthermore, the medical contact person over that 3-week term remained the same, thereby giving the patient a feeling of reliability and understanding.
A rehabilitation programme often includes a change in dietary habits, as patients receive whole-food nutrition on a regular schedule. As obesity, and also alcohol, poses trigger factors in PSO, dietary changes as well as physical exertion might have resulted in a better skin condition, leading to a greater improvement in pruritus.
Although many new systemic treatment options show quite promising results (34–36, 42, 43), conservative therapy remains indispensable, especially for patients with comorbidities and contraindications for systemic therapies. However, in an outpatient setting, some conservative therapeutic options, such as balneophototherapy, are limited due to the lack of availability of equipment, patient disabilities or interference with patients’ working hours. Therefore, rehabilitation programmes enable a continuous treatment for a period of at least 3 weeks, leading to good results concerning QoL, pruritus relief and psychological burdens. In case of insufficient recovery during that period and with the prospect of further stabilization with the respective therapeutic regimen, an extension of the rehabilitation programme may be required.
By differentiating the satisfaction of patients with our various therapeutic interventions, this study was able to show the importance of a multidisciplinary approach for patients with pruritus. The greatest satisfaction was achieved for balneophototherapy and phototherapy. As the majority of the current patients were diagnosed with PSO and AD, these findings go hand in hand with a clinical study published by the Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen in 2006 (37), which showed the therapeutic superiority of a combined balneophototherapy compared with phototherapy alone. As in an outpatient setting, the appliance of balneotherapy in patients with pruritus, especially with sulphuric well water, is extremely rare; and this suggests the benefit of an inpatient rehabilitation programme.
The high number of missing answers concerning satisfaction with the psychological care compared with the other therapeutic options may be explained by the missing medical indication, which was evaluated by the treating physician on admission and re-evaluated in the course of the rehabilitation. Still, even if there was a medical indication, some patients rejected a psychological consultation as they already received outpatient counselling or they generally rejected psychotherapeutic interventions. Society today still has taboos regarding psychological diseases and their need for treatment, leading to a stigmatization of patients in counselling. This may result in reluctance to accept psychological treatment. However, as patients with CP more frequently experience depression and anxiety than do healthy control groups, these psychological co-morbidities need to be addressed. Many of the current patients identified psychological triggers, such as emotional and inner tension. Furthermore, Schneider et al. (11) reported higher pruritus intensities as well as higher impairments in QoL in patients with psychosomatic diagnoses. Thus, in order to treat CP and provide access to psychological care, major steps towards destigmatization, as well as raising awareness in society and providing proper education, need to be ensured.
Based on the findings of this study, we propose introducing a pruritus-specific rehabilitation programme to the therapeutic regimen for patients with CP. Rehabilitation programmes in Germany have been established for various indications in several medical specialties. However, specific dermatological rehabilitation programmes are rarely initiated by general practitioners due to the lack of knowledge regarding the various dermatological indications for rehabilitation, ranging from oncological diseases over chronic inflammatory diseases to chronic wounds, pruritus and urticaria.
Study limitations
A limitation of this study is that the various specific treatment options were not administered in a randomized manner, because we aimed to meet patients’ needs as closely as possible. It therefore cannot be distinguished in which way the separate parts of the programme contributed to the therapeutic success. Only treatment satisfaction has been evaluated separately according to therapeutic option; however, with a questionnaire as yet untested for reliability and validity.
Another limitation of this study is the lack of a control group. Such a group should be ideally consist of patients receiving standard dermatological rehabilitation, or, alternatively, standard outpatient care. A factor that may have contributed to the positive patient outcome is that patients leave their everyday life for at least 3 weeks, which might contribute to an overall relaxation. In contrast, in regular dermatological inpatient facilities, the average hospital stay rarely exceeds 7 days. Furthermore, the largest subgroup consisted of patients with AD and PSO, which fall into group I (primary diseased, inflamed skin) according to the IFSI classification. Unfortunately, a comparison of this group with patients in group II (CP on non-inflamed skin) and group III (CP with secondary scratch lesions) was not possible due to small sample sizes. It is necessary to verify the results on a greater sample in order to allow statistical analysis of all subgroups.
Finally, this study did not investigate the long-term effects with a follow-up assessment some weeks after rehabilitation. Respective studies have already been initiated and are currently being conducted at the Department of Dermatology of the Medical Center Bad Bentheim.
Conclusion
Patients with CP experience reduced QoL, and CP may even lead to psychiatric disorders. The sole outpatient treatment often cannot provide the holistic biopsychosocial care plan that can be developed with patients in course of a medical rehabilitation. This study showed that, after a pruritus-specific rehabilitation programme, QoL was improved significantly, and psychological burden and pruritus were significantly reduced, indicating benefits for patients with CP. Considering the small number of patients in this study, it is necessary to reproduce these results with a larger patient collective at multiple study sites, including a control group, ideally with randomized group assignment and long-term follow-up.
ACKNOWLEDGEMENT
This paper contains parts of the doctoral thesis of the co-author Lisa Kok.
The authors have no conflicts of interest to declare.
REFERENCES
- Ständer S, Weisshaar E, Mettang T, Szepietowski JC, Carstens E, Ikoma A, et al. Clinical classification of itch: a position paper of the International Forum for the Study of Itch. Acta Derm Venereol 2007; 87: 291–294.
- Ständer S, Schäfer I, Phan NQ, Blome C, Herberger K, Heigel H, et al. Prevalence of chronic pruritus in Germany: results of a cross-sectional study in a sample working population of 11,730. Dermatology 2010; 221: 229–235.
- Matterne U, Apfelbacher CJ, Loerbroks A, Schwarzer T, Büttner M, Ofenloch R, et al. Prevalence, correlates and characteristics of chronic pruritus: a population-based cross-sectional study. Acta Derm Venereol 2011; 91: 674–679.
- Matterne U, Apfelbacher CJ, Vogelgsang L, Loerbroks A, Weisshaar E. Incidence and determinants of chronic pruritus: a population-based cohort study. Acta Derm Venereol 2013; 93: 532–537.
- Ständer S, Zeidler C, Augustin M, Bayer G, Kremer AE, Legat FJ, et al. S2k Guidelines for the diagnosis and treatment of chronic pruritus – update – short version. J Dtsch Dermatol Ges 2017; 15: 860–872.
- Grundmann SA, Stratmann E, Brehler R, Luger TA, Ständer S. Lactase deficiency: a potential novel aetiological factor in chronic pruritus of unknown origin. Acta Derm Venereol 2011; 91: 698–703.
- Ständer S, Blome C, Breil B, Bruland P, Darsow U, Dugas M, et al. [Erfassung von Pruritus – aktuelle Standards und Implikationen für die Praxis – Konsensuspaper der Initiative Pruritusparameter der Arbeitsgemeinschaft Pruritusforschung (AGP). Hautarzt 2012; 63: 521–522, 524–531.
- Kini SP, DeLong LK, Veledar E, McKenzie-Brown AM, Schaufele M, Chen SC. The impact of pruritus on quality of life: the skin equivalent of pain. Arch Dermatol 2011; 147: 1153–1156.
- Weisshaar E, Apfelbacher C, Jäger G, Zimmermann E, Bruckner T, Diepgen TL, et al. Pruritus as a leading symptom: clinical characteristics and quality of life in German and Ugandan patients. Br J Dermatol 2006; 155: 957–964.
- Lavery MJ, Stull C, Kinney MO, Yosipovitch G. Nocturnal pruritus: the battle for a peaceful night’s sleep. Int J Mol Sci 2016; 17: 425.
- Schneider G, Driesch G, Heuft G, Evers S, Luger TA, Ständer S. Psychosomatic cofactors and psychiatric comorbidity in patients with chronic itch. Clin Exp Dermatol 2006; 31: 762–767.
- Sheehan-Dare RA, Henderson MJ, Cotterill JA. Anxiety and depression in patients with chronic urticaria and generalized pruritus. Br J Dermatol 1990; 123: 769–774.
- Ferm I, Sterner M, Wallengren J. Somatic and psychiatric comorbidity in patients with chronic pruritus. Acta Derm Venereol 2010; 90: 395–400.
- Mazeh D, Melamed Y, Cholostoy A, Aharonovitzch V, Weizman A, Yosipovitch G. Itching in the psychiatric ward. Acta Derm Venereol 2008; 88: 128–131.
- Dalgard F, Lien L, Dalen I. Itch in the community: associations with psychosocial factors among adults. J Eur Acad Dermatol Venereol 2007; 21: 1215–1219.
- Halvorsen JA, Dalgard F, Thoresen M, Thoresen M, Bjertness E, Lien L. Itch and mental distress: a cross-sectional study among late adolescents. Acta Derm Venereol 2009; 89: 39–44.
- Weisshaar E, Szepietowski JC, Dalgard FJ, Garcovich S, Gieler U, Giménez-Arnau AM, et al. European S2k Guideline on Chronic Pruritus. Acta Derm Venereol 2019; 99: 469–506.
- Weisshaar E, Ständer S, Gieler U, Matterne U, Darsow U. Entwicklung eines deutschsprachigen Fragebogens zur Erfassung von chronischem Pruritus (AGP-Fragebogen) Hautarzt 2011; 62: 914–927.
- Reich A, Riepe C, Anastasiadou Z, Mędrek K, Augustin M, Szepietowski JC, et al. Itch assessment with visual analogue scale and numerical rating scale: determination of minimal clinically important difference in chronic itch. Acta Derm Venereol 2016; 96: 978–980.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI) – a simple practical measure for routine clinical use. Clin Exp Dermatol 1994; 19: 210–216.
- Zeidler C, Steinke S, Riepe C, Bruland P, Soto-Rey I, Storck M, et al. Cross-European validation of the ItchyQoL in pruritic dermatoses. J Eur Acad Dermatol Venereol 2019; 33: 391–397.
- Krause K, Kessler B, Weller K, Veidt J, Chen SC, Martus P, et al. German version of ItchyQoL: validation and initial clinical findings. Acta Derm Venereol 2013; 93: 562–568.
- Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006; 166: 1092–1097.
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001; 16: 606–613.
- Augustin M, Radtke MA, Zschocke I, Blome C, Behechtnejad J, Schafer I, et al. The patient benefit index: a novel approach in patient-defined outcomes measurement for skin diseases. Arch Dermatol Res 2009; 301: 561–571.
- Blome C, Augustin M. Evaluation des therapeutischen Nutzens aus Patientensicht: Der Patient Benefit Index (PBI) als Beispiel für zielorientierte Präferenz- und Outcome-Messung. Gesundh ökon Qual manag 2010; 15: 236–240.
- Foundation TR. The R Project for Statistical Computing. The R Foundation. 2021.
- Reich A, Heisig M, Phan NQ, Taneda K, Takamori K, Takeuchi S, et al. Visual analogue scale: evaluation of the instrument for the assessment of pruritus. Acta Derm Venereol 2012; 92: 497–501.
- Hongbo Y, Thomas CL, Harrison MA, Salek MS, Finlay AY. Translating the science of quality of life into practice: what do Dermatology Life Quality Index scores mean? J Invest Dermatol 2005; 125: 659–664.
- Hattori Y, Hattori K, Suzuki T, Matsuda N. Recent advances in the pathophysiology and molecular basis of sepsis-associated organ dysfunction: novel therapeutic implications and challenges. Pharmacol Ther 2017; 177: 56–66.
- Love EM, Marrazzo GA, Kini S, Veledar E, Chen SC. ItchyQoL bands: pilot clinical interpretation of scores. Acta Derm Venereol 2015; 95: 114–115.
- Frese T, Herrmann K, Sandholzer H. Pruritus as reason for encounter in general practice. J Clin Med Res 2011; 3: 223–229.
- Stinco G, Trevisan G, Piccirillo F, Pezzetta S, Errichetti E, di Meo N, et al. Pruritus in chronic plaque psoriasis: a questionnaire-based study of 230 Italian patients. Acta Dermatovenerol Croat 2014; 22: 122–128.
- Ständer S, Yosipovitch G, Legat FJ, Lacour JP, Paul C, Narbutt J, et al. Trial of nemolizumab in moderate-to-severe prurigo nodularis. N Engl J Med 2020; 382: 706–716.
- Ständer S, Spellman MC, Kwon P, Yosipovitch G. The NK1 receptor antagonist serlopitant for treatment of chronic pruritus. Expert Opin Investig Drugs 2019; 28: 659–666.
- He A, Alhariri JM, Sweren RJ, Kwatra MM, Kwatra SG. Aprepitant for the treatment of chronic refractory pruritus. BioMed Res Int 2017; 2017: 4790810.
- IQWiG. Nutzenbewertung der Balneophototherapie: Abschlussbericht N04/04: Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen; 2006 21.12.2006. Report No.: N04/04.
- Mollanazar NK, Koch SD, Yosipovitch G. Epidemiology of chronic pruritus: where have we been and where are we going? Curr Derm Rep 2015; 4: 20–29.
- AWMF online. German S1-Guideline “Stationäre dermatologische Rehabilitation” (extended version). [accessed 2022 Oct 30]. Available from: https://www.awmf.org/uploads/tx_szleitlinien/013-083l_S1_Stationaere-dermatologische-Rehabilitation_2020-04_01.pdf.
- Medical Service of the German Association of Health Insurance Companies, Guideline for Assessment of Rehabilitation according to § 282 SGB V: Vorsorge und Rehabilitation. July 2018. [accessed 2022 Oct 3]. Available from: https://www.mds-ev.de/fileadmin/dokumente/Publikationen/GKV/Begutachtungsgrundlagen_GKV/BGAVorsorge-Reha_18-07-02.pdf.
- German National Pension Fund. Statistics of the German National Pension Fund, Services for Medical Rehabilitation, Services for retention in professional life: details to services, duration, diagnoses, and professions 2018, Band 216; Berlin August 2019. [accessed 2022 Oct 3]. Available from: https://www.deutsche-rentenversicherung.de/SharedDocs/Downloads/DE/Statistiken-undBerichte/statistikpublikationen/statistikband_rehabilitation_2018.pdf?__blob=publicationFile&v=2.
- Müller S, Bieber T, Ständer S. Therapeutic potential of biologics in prurigo nodularis. Expert Opin Biol Ther 2022; 22: 47–58.
- Weisshaar E, Szepietowski JC, Bernhard JD, Hait H, Legat FJ, Nattkemper L, et al. Efficacy and safety of oral nalbuphine extended release in prurigo nodularis: results of a phase 2 randomized controlled trial with an open-label extension phase. J Eur Acad Dermatol Venereol 2022; 36: 453–461.
- Blome C, Augustin M, Siepmann D, Phan NQ, Rustenbach SJ, Ständer S. Measuring patient-relevant benefits in pruritus treatment: development and validation of a specific outcomes tool. Br J Dermatol 2009; 161: 1143–1148.
- Zeidler C, Pereira M, Dugas M, Augustin M, Storck M, Weyer-Elberich V, et al. The burden in chronic prurigo: patients with chronic prurigo suffer more than patients with chronic pruritus on non-lesional skin. J Eur Acad Dermatol Venereol 2021; 35: 738–743.
- Tsianakas A, Mrowietz M. Pruritus bei Psoriasis: Profil und Therapie. Hautarzt 2016; 67: 601–605.