ORIGINAL ARTICLE
Association of Chronic Urticaria with Psychological Distress: A Multicentre Cross-sectional Study
Samah TAWIL1,2, Carla IRANI3, Riwa KFOURY4, Soula ABRAMIAN5, Pascale SALAMEH1,4,6, Karsten WELLER7, Marcus MAURER7 and Khaled EZZEDINE8,9
1INSPECT-LB: Institut National de Santé Publique, Epidémiologie Clinique et Toxicologie, Beirut, Lebanon, 2Lebanese American University, School of medicine, Byblos, Lebanon, Beirut, 3Internal Medicine and Clinical Immunology, Hotel-Dieu de France Hospital, Saint Joseph University, Beirut, 4Faculty of Pharmacy and of Medical Sciences, Lebanese University, Hadath, Lebanon, 5Drug Information Center, Lebanese Order of Pharmacists, Beirut, Lebanon, 6Faculty of Medical School, University of Nicosia, Nicosia, Cyprus, 7Dermatological Allergology, Allergie-Centrum-Charité, Department of Dermatology and Allergy, Charité – Universitätsmedizin, Berlin, Germany, 8Department of Dermatology, Henri-Mondor University Hospital, Creteil, France, 9EA7379 Epiderm E (Epidemiology in Dermatology and Therapeutics Evaluation), UPEC-University, Paris-Est, Creteil, Creteil, France
Chronic urticaria is a debilitating disease that affects health-related quality of life, but few studies have evaluated its impact on psychological wellbeing. The aim of this study was to evaluate the quality of life of patients with chronic urticaria and determine its impact on their emotional and psychological wellbeing. A cross-sectional multicentre study of a cluster of 264 Lebanese patients visiting dermatology/immunology clinics was conducted between July 2018 and June 2020. The impact of chronic urticaria on quality of life was assessed using the Chronic Urticaria Quality of Life Questionnaire (CU-Q2oL) and Dermatology Life Quality Index (DLQI), as well as its consequences on mood changes using the Patient Health Questionnaire-9 (PHQ-9) and Beirut Distress Score 22 (BDS-22) scores. A multivariable analysis of covariates was performed to determine the effect of the triggering factors of urticaria on both CU-Q2oL and PHQ-9. A moderate negative correlation was found between Urticaria Control Test and quality of life scores as well as PHQ-9 and BDS-22 (p < 0.001). Patients with the lowest Urticaria Control Test score had the highest impairment in quality of life and depression scores. In conclusion, chronic urticaria compromises patients’ quality of life and emotional wellbeing. This distress is more pronounced when the disease is more severe.
Key words: chronic urticaria; quality of life; depression; psychological; correlation.
Citation: Acta Derm Venereol 2023; 103: adv00865. DOI https://doi.org/10.2340/actadv.v102.2939.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Sep 21, 2022; Epub ahead of print: Sep 21, 2022; Published: Feb 14, 2023
Corr: Samah Tawil, Drug Information Center, Lebanese Order of Pharmacists, Beirut, Lebanon. E-mail: samah.tawil@lau.edu; samahtawil@hotmail.com
INTRODUCTION
Chronic urticaria (CU) is characterized by the recurrent appearance of wheals and/or angioedema for more than 6 weeks. Most specialists concur that the discomfort associated with this disorder can pose a serious challenge that introduces a detrimental effect on patients’ quality of life (QoL) (1–5).
SIGNIFICANCE
Chronic urticaria is a dermatological disease that compromises the quality of life of patients, due to its debilitating symptoms. The recurrent pattern of this disease may have a detrimental effect on patients’ psychology, and many studies have demonstrated that patients may experience a prolonged psychological burden, such as depression. The evaluation of quality of life and psychological health is fundamental for a better assessment of disease progression and treatment efficacy. The results of this study demonstrate that urticaria is associated with an impairment in quality of life, which emphasizes the need for evaluation of the psychological health of patients visiting tertiary clinics.
Many studies have demonstrated that patients with CU experience a prolonged psychological burden, such as depression and anxiety (6–9). Others have shown that the symptoms of CU affect everyday life, limiting and impairing physical and emotional functioning, and act as an indirect burden on the life satisfaction of most of the patients (10–12). A recent survey showed that the majority of Canadian allergists reported that psychosocial parameters play a role in the pathogenesis of CU (13). In addition, a recent meta-analysis showed that the prevalence of any psychological condition among patients with CU was significantly higher than healthy subjects (14). Moreover, an online survey in Germany (ATTENTUS) reported the existence of a highly burdened population with CU outside specialized centres (15).
Thus, proper assessment of the health-related quality of life (HRQoL) using appropriate tools is important in estimating disease progression. Thus, many measures are used to assess QoL in patients with CU, such as the Dermatology Life Quality Index (DLQI) (16, 17), the Dermatology Quality of Life Scales (DQOLS) (18, 19) and the Chronic Urticaria Quality of Life Questionnaire (CU-Q2oL) (20–23).
Although urticaria currently represents a public health concern in the Middle East, the disease is still under-represented in this region. Only a few studies, such as the one conducted by Alzahrani et al., have shown that 41% of Saudi patients with CU experienced a large effect on their QoL (24, 25). Therefore, a more in-depth investigation of the increasing burden of CU may help address challenges to medical care in this region. The aim of the current study was to expand the use of a previously cross-validated version of CU-Q2oL along with other scales to evaluate the impairment of different aspects of life in patients with CU, as well as its impact on psychological wellbeing, mental and emotional health.
MATERIALS AND METHODS
Study design and sample size
A multi-centred cross-sectional study was conducted between July 2018 and June 2020, using a cluster sample of Lebanese patients with CU visiting dermatology and/or immunology clinics from all districts of Lebanon. Using Epi-info™ 7 for calculation of the sample size in which the primary endpoint is the worldwide prevalence of urticaria, a total number of 264 participants were required to participate in the study (taking into account a prevalence of 0.5–1% and a precision measure of 1.2% (26)). A total sample size of 295 participants was adopted to account for any missing value.
Patients were included if they were > 18 years old with a diagnosis of CU, defined as the daily or almost daily occurrence of hives, with or without angioedema, for at least 6 weeks. Patients were excluded if they had acute urticaria (< 6 weeks of hives), urticaria vasculitis or angioedema as a sole symptom, dermatitis or other skin diseases. The study also excluded patients who had a diagnosed psychiatric disorder, cognitive impairment, or central nervous system disease.
Study conduct
Participants were given the self-administered questionnaire in Arabic (the local language) and answered it independently. Completion of the questionnaire took approximately 10–15 min. Illiterate patients, geriatric patients, and those who had reading difficulties were interviewed by scientific researchers who had received training in advance. Patients were asked to provide their contact information for further follow-up in case of any missing information. They provided information on their socio-demographic characteristics, such as sex, age, highest educational level, type of employment, income, marital status, and number of individuals per household, as well as their medical information, such as the main reason for the doctor’s visit, current and past medical history, any family history of dermatological diseases, symptoms of the disease and the main triggering factors, including food, medications, seasonal pollen, cold, heat, contact substances, insect bites, and animals. Each patient was asked to complete the questionnaire, which included several severity and QoL scales, and to recall appropriate information.
Description of the Urticaria Control Test
The Urticaria Control Test (UCT) is a 4-item questionnaire designed to assess the level of disease control among patients with CU within the past 4 weeks. Items assess the level of suffering due to the physical symptoms of urticaria, its impact on QoL, the effect of treatment on urticaria symptom control, and overall perceived urticaria control. Higher scores on the UCT are indicative of better disease control. The total score ranges from 0 (no disease control) to 16 (complete disease control). A score less than 12 indicates uncontrolled symptoms and a score less than 8 indicates very poor symptom control (27). The Arabic version of the UCT was previously linguistically validated to screen Arabic-speaking patients for poorly vs well-controlled disease (28).
Description of the Chronic Urticaria Quality of Life Questionnaire, and the Dermatology Life Quality Index
The CU-Q2oL comprises 23 items categorized into 6 domains: pruritus (n = 2), impact on life activities (n = 6), problems with sleep (n = 5), limits (n = 3), looks (n = 5), and swelling (n = 2). Each item is scored on a 5-point Likert scale, ranging from 1=”not at all” to 5=”very much”. The individual items are summed up to generate the overall CU-Q2oL score, which is then converted to a 0–100 scale. Higher scores indicate greater QoL impairment (29). It is noteworthy that a previously cross-validated Arabic version of CU-Q2oL was used in our study for data collection (30).
The DLQI is a HRQoL questionnaire for dermatological diseases that comprises 10 questions corresponding to 6 domains: symptoms and feeling, daily activities, leisure, work and school, personal relationships, and treatment (23). It is available in 55 languages, including Arabic (31–33). Like the CU-Q2oL, the Arabic DLQI was previously used for assessment of the QoL of a sample of Lebanese patients with CU (30). The total DLQI score ranges from 0 to 30. The answers are scored on a 4-point Likert scale (0 for not at all and 3 for very much). Higher scores indicate higher QoL impairment.
Description of Patient Health Questionnaire-9 (PHQ-9) and Beirut Distress Score 22 (BDS-22)
The PHQ-9 includes 9 items, which focus on the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-V) for Major Depressive Disorder (34). Each item of PHQ-9 is scored on a scale of 0–3 (0 = not at all; 1 = several days; 2 = more than a week; 3 = nearly every day). The PHQ-9 total score ranges from 0 to 27 (scores of 0–9 are classified as mild depression; 10–14 as moderate depression; 15–19 as moderately severe depression; ≥ 20 as severe depression) (35). PHQ-9 validity was well established in a sample of Lebanese adults and it was shown to be a sensitive measure of depressive symptoms (36).
The BDS-22 is a 22-item questionnaire that differentiates among core symptoms of depression, anxiety, and perceived stress. It is a reliable and valid tool used for the assessment of psychological distress and mental health perception in the general population. It includes 6 factors, reflecting depressive symptoms, demotivation, psychosomatic symptoms, mood deterioration, intellectual inhibition and anxiety. The 22 items are answered on a Likert scale from 0 to 3 (0 never, 1 sometimes, 2 often, and 3 always) with a possible score from 0 to 66. The score is then summed up by adding up all the items and the higher the score, the greater the risk of psychological distress is (37, 38).
Data processing and statistical methods
All data analyses and statistical processes were performed using SPSS version 23 (IBM SPSS Software, Chicago, IL, USA). Continuous measures were summarized by means and standard deviation (SD) or by medians and interquartile range (IQR), where appropriate; categorical measures were summarized by percentage and 95% confidence intervals (95% CI).
Continuity correction χ2 tests were used for the comparison of categorical variables between groups. The conformity of continuous variables to normal distribution was evaluated using visual histogram and probability graphs. Independent-samples t-test and analysis of variance (ANOVA) were used for the comparison of normally distributed variables. The variation of different scores among 3 different disease control levels (classified as very poorly controlled (UCT<8), poorly controlled (UCT 8–11), or well-controlled (UCT≥12) was investigated using the Kruskal–Wallis test.
The relationship between disease control and all remaining scores were evaluated using Pearson’s or Spearman’s correlation analysis. Correlation coefficients of < 0.3, 0.3–0.6, and > 0.6 were considered to indicate weak, moderate, and strong correlations, respectively (39). Multivariable analysis of variance (MANCOVA) was performed, where CU-Q2oL and PHQ-9 were the dependent variables, age and sex the covariates and socio-demographics and risk factors the independent variables in order to assess the effect of the different factors associated with urticaria on both QoL and depression. A p-value ≤ 0.05 was considered to be statistically significant.
Ethical considerations
The Lebanese University ethics committee waived the need for approval, as the study was observational and respected participants’ confidentiality. Patients provided their oral informed consent before participation.
RESULTS
Patients socio-demographic and disease characteristics
A total of 295 patients with CU participated in the study. The mean age was 35.7 ± 11.33 years (range 18–81). The female:male ratio was 161:134 (female: 54.6%). The majority of the patients did not have any positive family history of dermatological disease (75.8%). The final diagnosis was mainly confirmed an allergist/immunotherapist in most of the cases (87.1%), or dermatologist (8.1%), or physicians with other specialties, such as ear nose and throat (ENT), paediatrics, cardiovascular, pulmonologist, etc. (4.8%). Both spontaneous and induced types of urticaria were encountered, including physical (17.3% contact urticaria and 1.7% delayed pressure urticaria,) and non-physical (35.9% pollen allergy; 10.5% dust allergy; 2.4% solar urticaria; 2% heat-induced urticaria; and 1.4% cold-induced urticaria). More details about socio-demographic and disease characteristics are listed in Table I.
Patients’ disease severity, quality of life and psychological distress assessment
The mean score of UCT in all patients with CU had a value of 9.3 ± 3.12, indicating a moderate disease control. The means of both DLQI and CU-Q2oL were 7.71 ± 5.82 and 40.06 ± 15.4 respectively. As for the scores measuring the psychological distress, the mean scores had values of 24.19 ± 14.5 and 12.5 ± 7.17 for BDS-22 and PHQ-9 scores, respectively, which indicate a mild depression level. More details about these scores are summarized in Table II.
Association between patient’s quality of life and urticaria control. Patients were classified into 3 UCT categories: 1: very poorly controlled (score < 8); 2: poorly controlled (score 8–11); and 3: well controlled (score ≥ 12). When comparing the DLQI scores among the 3 groups, no statistically significant difference in median scores was shown between the patients with a UCT score < 8 and UCT score 8–11. However, the well-controlled group had the lowest DLQI median score (4.00 ± 4.75; p < 0.001; Fig. 1a). The same trend was also seen in the variation of CU-Q2oL median scores, where no statistically significant differences were detected between the very poorly controlled and poorly controlled group, whereas patients in the well-controlled group showed the lowest CU-Q2oL median score (34.00 ± 14.00; p < 0.001; Fig. 1b).
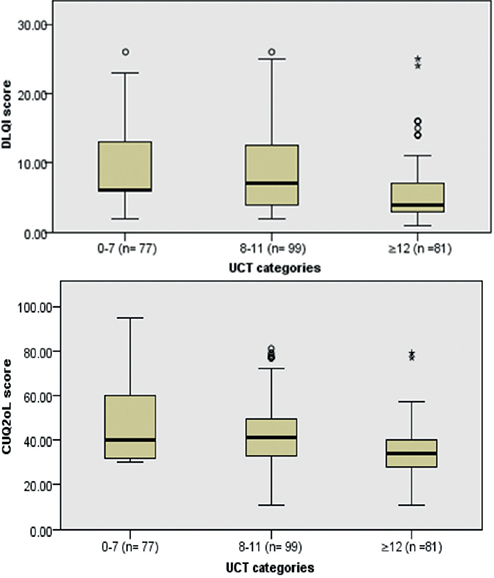
Fig. 1. (a) Dermatology Life Quality Index (DLQI) median scores differences among Urticaria Control Test (UCT) categories; p < 0.001. (b) Chronic Urticaria-Quality of Life Index (CUQ2oL) median scores differences among UCT categories; p < 0.001* and °outlier values.
Association between patient’s psychological health and urticaria control. PHQ-9 and BDS-22 median scores differed significantly among the 3 UCT categories. Patients with UCT scores < 8 had the highest median scores of PHQ-9 and BDS-22 (17.5 ± 8.75 and 39.0 ± 18.5, respectively) when compared with poorly controlled (PHQ-9=16.0 ± 6.75 and BDS-22=27.5 ± 10.0; p < 0.001) and well-controlled groups (PHQ-9=4 ± 9.75 and BDS-22=13 ± 8.25; p < 0.001; Fig. 2a and 2b)
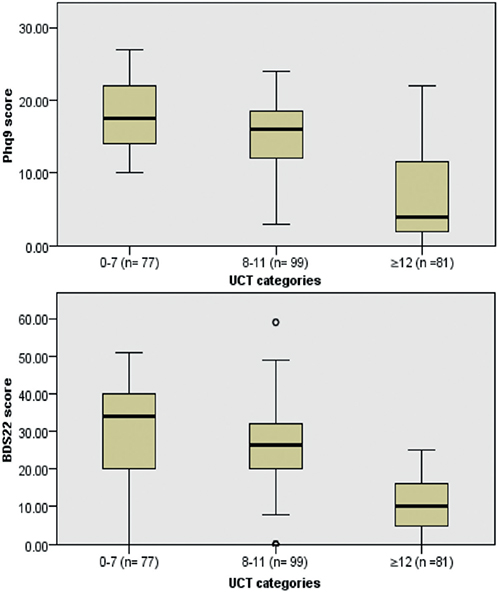
Fig. 2. (a) Patient Health Questionnaire (PHQ-9) median scores differences among Urticaria Control Test (UCT) categories; p < 0.001. (b) Beirut Distress Score (BDS-22) median scores differences among UCT categories; p < 0.001. °Outlier value.
Correlation between quality of life and psychological distress scores and urticaria control
The HRQoL scores were shown to have a moderate negative correlation with UCT; i.e. a moderate correlation was found between DLQI total score and UCT (rho = –0.51, p < 0.001) and CU-Q2oL total score and UCT (rho = –0.55, p < 0.001), respectively. The same was also noticed between the psychological distress scores and UCT (PHQ-9: rho = –0.55 and BDS-22: rho = –0.61, p < 0.001). More details about correlation measurements are shown in Fig. 3.
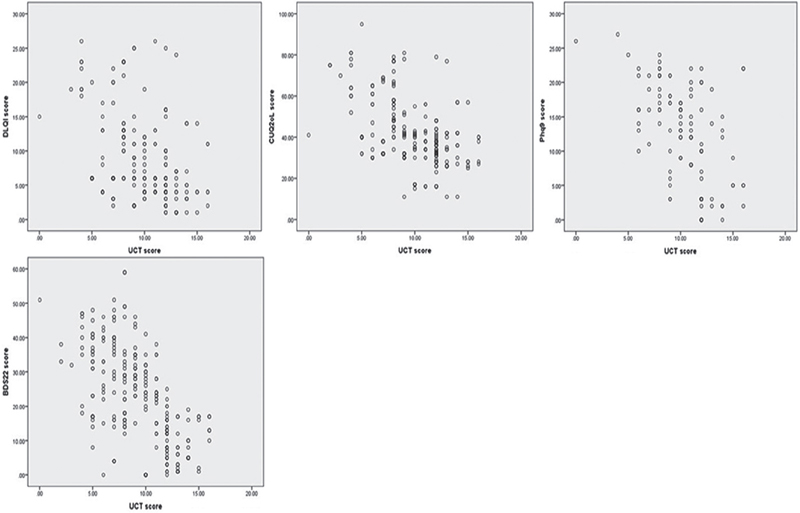
Fig. 3. (a) Correlation between Dermatology Life Quality Index (DLQI) total score and Urticaria Control Test (UCT); rho=–0.51; p<0.001. (b) Correlation between Chronic Urticaria-Quality of Life Index (CUQ2oL) total score and UCT; rho=–0.55; p < 0.001. (c) Correlation between Patient Health Questionnaire (PHQ-9) total score and UCT; rho=–0.55; p < 0.001. (d) Correlation between Beirut Distress Score (BDS-22) total score and UCT; rho=–0.61; p < 0.001.
Distribution of scores among different categories of clinical characteristics
MANCOVA analysis of both CU-Q2oL and PHQ-9 scores was performed among different categories of sociodemographic characteristics and risk factors, using sex and age as covariates. The model did not show any significant difference in covariance, and all assumptions of normality, linearity and correlation were taken into account. Variables such as spontaneous urticaria and emotional stress were associated with an increase in CU-Q2oL. Moreover, having a lower level of education than a university degree was associated with an increase in PHQ-9 score, indicating higher depression than other educational categories. More details about score differences are shown in Table III.
DISCUSSION
This study was conducted to evaluate the burden of CU on HRQoL and psychological wellbeing in real-life clinical practice. The demographic characteristics of the patients were comparable with the findings of previous publications (40, 41) and occurrence of CU was mostly detected in the category of 20-40 years old, which is consistent with the previous findings (15, 26).
This study evaluated disease control of urticaria using UCT score, and revealed that approximately 68.6% of patients with CU had uncontrolled symptoms. Also, a moderate negative correlation was shown between UCT scores and each of CU-Q2oL and DLQI. This is similar to the findings of the study by Weller et al. (27), in which it was reported that the screening accuracy of the UCT was high in screening disease severity. It is notable that similar findings were reported by other authors who highlighted a higher DLQI score among patients with high UCT score (42). Although a change in UCT may indicate a change in the patient’s HRQoL, as seen in the current study similar to the results of the study by Stull et al. (43), the moderate correlation can be explained by the fact that disease control and HRQoL are different concepts. HRQoL is influenced not only by the disease itself, but also by additional factors, such as sleep deprivation and other socio-demographic features.
The current findings have proven that CU affects the QoL of almost half of the affected patients (50.3%). Unsurprisingly, similar studies revealed that CU compromises the patients’ QoL underlining the need to provide the most appropriate treatment (44–46). In a study conducted by Alzahrani et al in Saudi Arabia, it was proven that disease severity had markedly affected self-perception and mental status (25). Moreover, another French study showed that CU impaired the QoL of the affected patients and underlined the fact that this impairment is often underestimated (47).
In addition, the current study has shown that patients with CU experienced depression and psychological distress, which was more serious when urticaria was less controlled. This was underlined by the study by Sperber et al., who performed a psychological assessment to 19 patients with chronic inducible urticaria and found that these patients had significantly higher depression and anxiety scores compared with the control group (48).
The goal of this study was to provide proof of concept for the potential utility of PHQ-9 and BDS-22 scales in allergy/immunology clinics in the management of patients with CU with regards to depression and psychological distress. Given the unpredictable nature of individual urticaria episodes, it is not surprising to find an association between CU and depression (9, 49).
The current study is similar to previous ones in which no impact on QoL was associated with urticaria in terms of sex differentiation, income, age, and marital status, etc. (47, 50). Only stress and the spontaneous type of urticaria were associated with an increase in CU-Q2oL score, while lower educational level was associated with more depression. This proves that stress may influence negatively the QoL of the patients affected with urticaria. In fact, CU is known to be a psycho-dermatological disorder. It was proven that factors such as stress can affect exacerbation of urticaria wheals, and psychological distress may be related to itch intensity (51, 52). Furthermore, it was shown that the patients who did not know the exact trigger behind their disease had worse impact on their QoL than those who did. This finding is logical, since it is usually easier to cope with the disease when the cause is well defined and avoidable. Regarding educational level, it is predictable to notice that patients with a lower educational level feel more depressed than those with more diplomas. Usually, people with a higher educational level have more employment chances and a better lifestyle, as seen in the study by Baumann et al. (53) on 355 students from Europe. It was concluded that the psychological dimension of QoL was positively associated with academic skills and more knowledge regarding employability and activities of daily living.
The current study provides the basis for further research projects regarding CU, which are of particular interest as this disease is often poorly controlled. Further research into poor disease control by the development of simple diagnostic tools to help patients become more integrated in the treatment process. Moreover, motivational interviewing technique and disease registries may help achieve better outcomes in disease management. It is essential for all healthcare professionals to examine further the psychological aspects of living with CU. Additional research on referral for counselling could be valuable, as this is something that is not covered in depth in the available guidelines.
To our knowledge, this is one of few studies to evaluate the impact of urticaria on QoL and psychological health. In addition, the use of the CU-Q2oL, which is a specific instrument to evaluate the HRQoL in CU, added power to this study, in contrast to most studies that used generic questionnaires for dermatological diseases, such as the DLQI alone. In addition, this study aimed to evaluate the impact of CU on daily activities in a multicentre setting, which enhances the generalizability of the results. Moreover, the fact that all patients with all types of CU were included improved the representability of the current sample. Despite this, some limitations should be considered. First, the retrospective collection of data may have increased the risk of recall bias. In addition, all outcome measures were self-reported, which may be open to bias such as social desirability. Furthermore, this study was uncontrolled in a small sample of patients with CU, which would have reduced power in detecting significant differences in QoL and depression scores. Moreover, researchers did not have access to medical records beyond initial screening for eligibility. As such, there is a lack of knowledge regarding changes in medical care and their possible effects on outcome measures or impact on QoL.
In conclusion, CU compromises the QoL of patients due to its debilitating symptoms, which can last for years. The evaluation of QoL is fundamental to better assess disease progression and treatment efficacy. The current findings call for psychological health evaluation of patients with CU in routine clinical practice.
ACKNOWLEDGEMENTS
Conflicts of interest: The authors declare that IC is a full–time physician practicing at the Internal Medicine and Clinical Immunology Department at Hôtel-Dieu de France hospital, Beirut. Otherwise, the authors have no other conflicts of interest or financial support from pharmaceutical companies to declare in relation to this work.
REFERENCES
- Zuberbier T, Aberer W, Asero R, Bindslev-Jensen C, Brzoza Z, Canonica GW, et al. The EAACI/GA(2) LEN/EDF/WAO Guideline for the definition, classification, diagnosis, and management of urticaria: the 2013 revision and update. Allergy 2014; 69: 868–887.
- Ferrer M. Epidemiology, healthcare, resources, use and clinical features of different types of urticaria. Alergologica 2005. J Investig Allergol Clin Immunol 2009; 19: 21–26.
- Balp MM, Khalil S, Tian H, Gabriel S, Vietri J, Zuberbier T. Burden of chronic urticaria relative to psoriasis in five European countries. J Eur Acad Dermatol Venereol 2018; 32: 128–290.
- Murota H, Kitaba S, Tani M, Wataya-Kaneda M, Azukizawa H, Tanemura A, et al. Impact of sedative and non-sedative antihistamines on the impaired productivity and quality of life in patients with pruritic skin diseases. Allergol Int 2010; 59: 345–354.
- Grob JJ, Revuz, J, Ortonne JP, Auquier P, Lorette G. Comparative study of the impact of chronic urticaria, psoriasis and atopic dermatitis on the quality of life. Br J Dermatol 2005; 152: 289–295.
- Balp MM, Vietri J, Tian H, Isherwood G. The impact of chronic urticaria from the patient’s perspective: a survey in five European countries. Patient 2015; 8: 551–558.
- Weldon DR. Quality of life in patients with urticaria. Allergy Asthma Proc 2006; 27: 96–99.
- Baiardini I, Giardini A, Pasquali M. Dignetti P, Guerra L, Specchia C, et al. Quality of life and patients’ satisfaction in chronic urticaria and respiratory allergy. Allergy 2003; 58: 621–23.
- Engin B, Uguz F, Yilmaz E, Ozdemir M, Mevlitoglu I. The levels of depression, anxiety and quality of life in patients with chronic idiopathic urticaria. J Eur Acad Dermatol Venereol 2008; 22: 36–40.
- Staubach P, Eckhardt-Henn A, Dechene M, Vonend A, Metz M, Magerl M, et al. Quality of life in patients with chronic urticaria is differentially impaired and determined by psychiatric comorbidity. Br J Dermatol 2006; 154: 294–298.
- Papadopoulou N, Kalogeromitro SD, Staurianea NG, Tiblalexi D, Theoharides TC. Corticotropin-releasing hormone receptor-1 and histidine decarboxylase expression in chronic urticaria. J Invest Dermatol 2005; 125: 952–955.
- Lindsay K, Goulding J, Solomon M, Broom B. Treating chronic spontaneous urticaria using a brief ‘whole person’ treatment approach: a proof-of-concept study. Clin Translational Allergy 2015; 5:40.
- Ben-Shoshan M, Clarke A, Raz A. Psychosocial factors and the pathogenesis of chronic hives: a survey of Canadian physicians. J Allergy Therapy 2012; 3:1–2.
- Konstantinou GN, Konstantinou GN. Psychiatric comorbidity in chronic urticaria patients: a systematic review and meta-analysis. Clin Translational Allergy 2019; 9:42.
- Maurer M, Staubach P, Raap U, Richter-Huhn G, Baier-Ebert M, Chapman-Rothe N. ATTENTUS, a German online survey of patients with chronic urticaria highlighting the burden of disease, unmet needs and real-life clinical practice. Br J Dermatol 2016; 174: 892–894.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI) – a simple practical measure for routine clinical use. Clin Exp Dermatol 1994; 19: 210–216.
- Lewis V, Finlay AY. 10 years’ experience of the Dermatology Life Quality Index (DLQI). J Invest Dermatol Symp Proc 2004; 9: 169–180.
- Salek MS, Jung S, Brincat-Ruffini LA, MacFarlane L, Lewis-Jones MS, Basra MK, et al. Clinical experience and psychometric properties of the Children’s Dermatology Life Quality Index (CDLQI), 1995–2012. Br J Dermatol 2013; 169: 734–759.
- Tondury B, Muehleisen B, Ballmer-Weber BK, Hofbauer G, Schmid-Grendelmeier P, French L, et al. The Pictorial representation of Iilness and self-measure (PRISM) instrument reveals a high burden of suffering in patients with chronic urticaria. J Investig Allergol Clin Immunol 2011; 21: 93–100.
- Baiardini I, Fasola S, Maurer M, Weller K, Canonica GW, Braido F. Minimal important difference of the Chronic Urticaria Quality of Life Questionnaire (CU-Q2oL). Allergy 2019; 74: 2542–2544.
- Brzoza Z, Badura-Brzoza K, Młynek, A, Magerl M, Baiardini I, Canonica GW, et al. Adaptation and initial results of the polish version of the GA²LEN chronic urticaria quality of life questionnaire (CU-Q2oL). J Dermatol Sci 2011; 62: 36–41.
- Młynek A, Magerl M, Hanna M, Lhachimi S, Baiardini I, Canonica GW, et al. The German version of the chronic urticaria quality-of-life questionnaire (CU-Q2oL): factor analysis, validation and initial clinical findings. Allergy 2009: 64; 927–936.
- Baiardini I, Pasquali M, Braido F, Fumagalli F, Guerra L, Compalati E, et al. A new tool to evaluate the impact of chronic urticaria on quality of life: chronic urticaria quality of life questionnaire (CU-QoL). Allergy 2005; 60: 1073–1078.
- Alzahrani NS, Alshammari MA, Alshaer ZA, Alfouwais NM, Alhunaki RN, Albasi AA, et al. Chronic idiopathic urticaria impacts on patients’ quality of life. Saudi Ar Int J Sci & Eng Res 2017; 8: 1246–1247.
- Maurer M, Ortonne JP, Zuberbier T. Chronic urticaria: an internet survey of health behaviors, symptom patterns and treatment needs in European adult patients. Br J Dermatol 2009; 160: 633–641.
- Maurer M, Weller K, Bindslev-Jensen C, Giménez-Arnau A, Bousquet PJ, Bousquet J, et al. Unmet clinical needs in chronic spontaneous urticaria: a GA²LEN task force report. Allergy 2011; 66:317–330.
- Weller K, Groffik A, Church MK, Hawro T, Krause K, Metz M, et al. Development and validation of the urticarial control test: a patient-reported outcome instrument for assessing urticarial control. J Allergy and Clin Imm 2014; 133: 1365–1372.
- Irani C, Hallit S, Weller K, Maurer M, El Haber C, Salameh P. Chronic urticaria in most patients is poorly controlled: Results of the development, validation, and real life application of the arabic urticaria control test. Saudi Med J 2017; 38: 1230–1236.
- Lennox RD, Leahy MJ. Validation of the Dermatology Life Quality Index as an outcome measure for urticaria-related quality of life. Ann Allergy Asthma Immunol 2004; 93: 142–146.
- Tawil S, Irani C, Kfoury R, Salameh P, Baiardini I, Weller K, et al. The Arabic Urticaria Activity Score and Chronic Urticaria Quality of Life Questionnaire: validation and correlations. Int J Dermatol 2020; 59: 893–901.
- Lambachahab FE, Abouqqal R, Hassam B, Ismaili N. The Arabic version of the dermatology life quality index for Morocco: psychometric properties in psoriasis. J Am Ac Derm 2010; 62: 138–142.
- Basra MK, Fenech R, Gatt RM, Salek MS, Finlay AY. The Dermatology Life Quality Index 1994-2007: a comprehensive review of validation data and clinical results. Br J Dermatol 2008; 159: 997–1035.
- Eltaher SM, Araby EM. Health related quality of life in patients with vitiligo. Egypt J Comm Medi 2015; 33: 77–83.
- American Psychiatric Association, 2022. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision (DSM-5-TR™). Washington, DC: American Psychiatric Press.
- Kroenke K, Spitzer RL, Williams JB, Lowe B. The patient health questionnaire somatic, anxiety, and depressive symptom scales: a systematic review. Gen Hosp Psychiatry 2010; 32: 345–359.
- Sawaya H, Atoui M, Hamadeh A, Zeinoun P, Nahas Z. Adaptation and initial validation of the Patient Health Questionnaire – 9 (PHQ-9) and the Generalized Anxiety Disorder – 7 Questionnaire (GAD-7) in an Arabic speaking Lebanese psychiatric outpatient sample. Psych Res 2016; 239: 245–252.
- Abou Abbas L, Salameh P, Mansour Z, Nasser Z, Elias E, Godin I. Development and initial validation of a brief scale for assessing psychological distress in obese adults. Clin Epidemiol Global Health 2016; 4: 16–22.
- Barbour B, Saadeh N, and Salameh P. Psychological distress in Lebanese young adults: constructing the screening tool “BDS-22”. Int J Culture and Mental Health 2012; 5: 94–108.
- Nunnaly JC, Bernstien IH. Psychometric theory. 3rd ed. NewYork: Mc Graw-Hill; 1994.
- Takeuchi S, Esaki H, Furue M. Epidemiology of atopic dermatitis in Japan. J Dermatol 2014; 41: 200–204.
- Kubota K, Kamijima Y, Sato T, Ooba N, Koide D, Iizuka H, et al. Epidemiology of psoriasis and palmoplantar pustulosis: a nationwide study using the Japanese national claims database. Br Med J Open 2015; 5: e006450.
- Itakura A, Tani Y, Kaneko N, Hide, M. Impact of chronic urticaria on quality of life and work in Japan: Results of a real-world study. J Derm 2018; 45: 963–970.
- Stull DE, McBride D, Houghton K, Finlay AY, Gnanasakthy A, Balp MM. Assessing changes in chronic spontaneous/idiopathic urticaria: comparisons of patient-reported outcomes using latent growth modeling. Adv Ther 2016; 33: 214–224.
- Dias GC, Pires GV, Do Valle SR, Dortas SD, Levy S, França AT, et al. Impact of chronic urticaria on the quality of life of patients followed up at a university hospital. An Bras Dermatol 2016; 91: 754–759.
- Yun J, Katelaris CH, Weerasinghe A, Adikari DB, Ratnayake C. Impact of chronic urticaria on the quality of life in Australian and Sri Lankan populations. Asia Pac Allergy 2011; 1: 25–29.
- Heng JK, Koh LJ, Toh MP, Aw DC. A study of treatment adherence and quality of life among adults with chronic urticaria in Singapore. Asia Pac Allergy 2015; 5: 197–202.
- Revuz JJ, Ortonne JP, Auquier P, Lorette G. Comparative study of the impact of chronic urticaria, psoriasis and atopic dermatitis on the quality of life. Br J Derm 2005; 152: 289–295.
- Sperber J, Shaw J, Bruce S. Psychological components and the role of adjunct interventions in chronic idiopathic urticaria. Psychother Psychosom 1989; 51: 135–141.
- Özkan M, Oflaz SB, Kocaman N, Özşeker F, Gelincik A, Büyüköztürk S, et al. Psychiatric morbidity and quality of life in patients with chronic idiopathic urticaria. Ann Allergy Asthma Immunol 2007; 99: 29–33.
- Zuberbier T, Balke M, Worm M, Edenharter G, Maurer M. Epidemiology of urticaria: a representative cross-sectional population survey. Clin Exp Dermatol 2010; 35: 869–873.
- Niemeier V, Nippesen M, Kupfer J, Schill WB, Gieler U. Psychological factors associated with hand dermatoses: which subgroup needs additional psychological care? Br J Dermatol 2002; 146: 1031–1037
- Verhoeven L, Kraaimaat F, Duller P, van de Kerkhof P, Evers A. Cognitive, behavioral, and physiological reactivity to chronic itching: analogies to chronic pain. Int J Behav Med 2006; 13: 237–243
- Baumann M, Ionescu I, Chau N. Psychological quality of life and its association with academic employability skills among newly-registered students from three European faculties. BMC Psychiatry 201; 11: 63.