Dupilumab (DUP) is a human monoclonal antibody used as a treatment foratopic dermatitis (AD), which blocks interleukin-4 and interleukin-13 signalling. According to Japanese guidelines, temporary discontinuation of DUP should be considered approximately 6 months after remission. However, it is unclear whether patients with AD can maintain remission after discontinuation of DUP in clinical practice. Therefore, the aim of this study was to retrospectively investigate the clinical course of patients treated with DUP for AD in our hospital who discontinued DUP after remission of AD.
MATERIALS AND METHODS
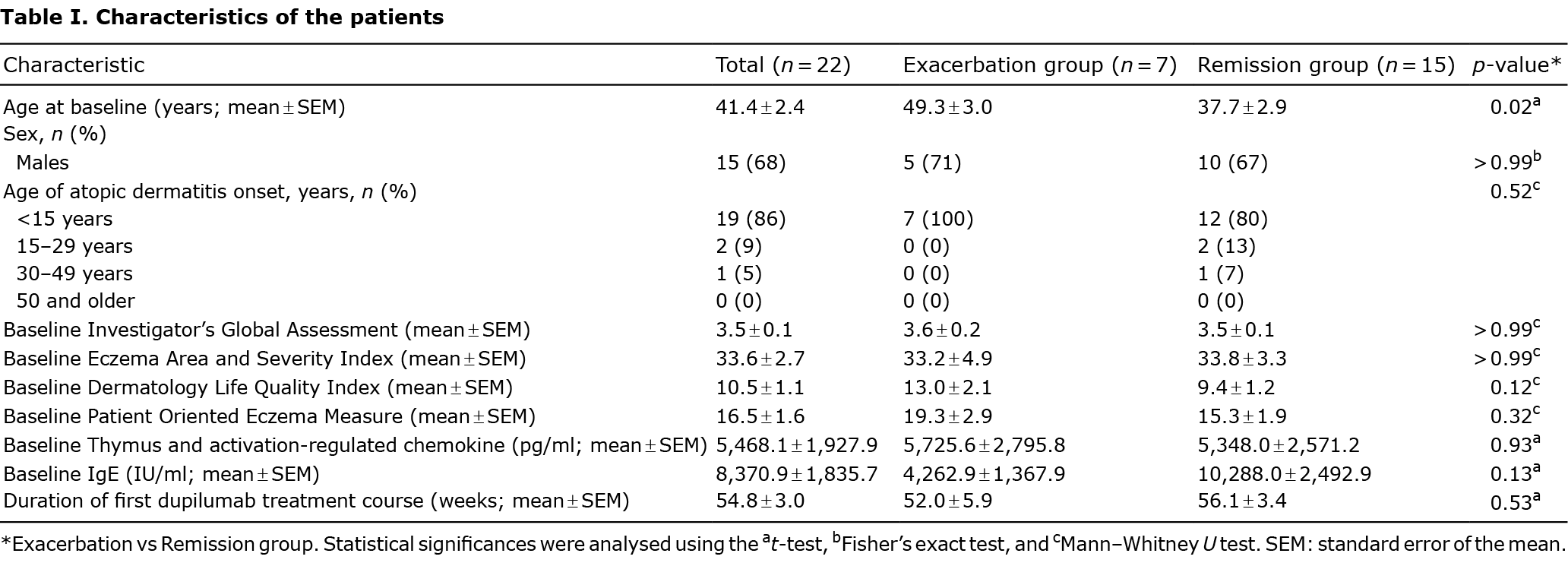
This study was conducted in compliance with the guidelines of the Declaration of Helsinki and with the approval of the ethics committee of Hyogo College of Medicine. Of 109 AD patients (63 males and 46 females) who commenced DUP treatment in our hospital between April 2018 and July 2020,the study included 22 patients (15 males and 7 females) who had follow-ups for at least 16 weeks after discontinuation of DUP following > 6 months’ remission (Fig. S1). Remission was defined as a condition in which disease of patients with AD stabilized with proactive therapy of topical steroids or calcineurin inhibitors. All patients received 600 mg DUP as a loading dose and 300 mg every 2 weeks thereafter, in addition to induction therapy with daily application or proactive therapy with intermittent application of topical steroids or calcineurin inhibitors during and after DUP treatment. Very strong-class or strong-class topical steroids were used mainly for the trunk and limbs, and medium-class topical steroids or calcineurin inhibitors for the head and neck. Emollients were used for the whole body, and oral antihistamines were used when needed. The amount and frequency of the topical applications were determined according to the proactive therapy recommended in Japanese AD guidelines (1). Data were collected on the clinical characteristics of the patients assessed using the Investigator’s Global Assessment (IGA), Eczema Area and Severity Index (EASI), Dermatology Life Quality Index (DLQI), Patient Oriented Eczema Measure (POEM) and serum levels of thymus and activation-regulated chemokine (TARC) and IgE at the following times: first administration of DUP; 16 weeks after first administration; DUP dosing discontinuation (> 6 months after achievement of remission); 4, 8 and 16 weeks after DUP dosing discontinuation; initiation of DUP re-treatment; and 16 weeks after DUP re-initiation. Changes in the clinical characteristics of the patients from the time of first administration, as assessed by the IGA, EASI, DLQI and POEM and serum levels of TARC and IgE, were calculated as percentages. Missing values were imputed using the last observation carried forward (LOCF) method. For the baseline clinical characteristics of the patients with AD, the study compared the patient group who requested re-treatment with DUP because their disease worsened with intermittent application of topical steroids or calcineurin inhibitors and required daily application after discontinuation of DUP (exacerbation group) with the other group who did not request resumption of DUP treatment because their disease stabilized with intermittent application of treatment (remission group). The t-test, Fisher’s exact test and Mann–Whitney U test were used for comparing the baseline clinical characteristics.
RESULTS
The probability of long-term biologic-free remission in the 22 patients with AD who discontinued DUP following remission is shown in a Kaplan–Meier curve (Fig. 1). The exacerbation group with 7 patients resumed DUP 15.6 ± 2.4 weeks (mean ± standard error of the mean (SEM)) after discontinuation. The remission group with 15 patients remained in remission for 40.5 ± 4.6 weeks (mean ± SEM) after discontinuation. The baseline clinical characteristics of the AD patients are summarized in Table I. None of the 22 patients had previously been treated with systemic treatments. The patient’s age was significantly higher in the exacerbation group than in the remission group. Fig. S2 shows the rate of change in the clinical characteristics of the patients, as assessed by the IGA, EASI, DLQI and POEM and serum levels of TARC and IgE in the exacerbation and remission groups. In the exacerbation group, the values except for IgE worsened after discontinuation of DUP and improved after initiation of DUP re-treatment. However, all values improved gradually over the long term in the remission group. Detailed data on the patients in the exacerbation group and remission group are shown in Table SI.

DISCUSSION
In Japan, patients typically pay 30% of their medical expenses. A previous study reported that financial considerations were one of the primary reasons for discontinuation of DUP in Japanese patients (n = 11/13) (2). However, the prognosis after discontinuation of DUP is unclear. In a real-world clinical practice study, 6 patients with AD had responded to DUP after a mean of 23 weeks and remained in remission for a mean of 12 weeks after discontinuation of DUP, but no further follow-up was reported (3). In a clinical trial of patients with AD treated with DUP, the disease worsened soon after discontinuation of DUP (4), but it was a large study and the details of each patient were unknown. The current study investigated whether patients with AD treated in our hospital maintained remission after discontinuation of DUP.
The results show that more than half of the patients who discontinued DUP treatment achieved long-term remission (40.5 ± 4.6 weeks; n = 15/22). In contrast, in patients with AD treated with cyclosporine, treatment discontinuation was associated with relapse 2 weeks later in approximately 50% of patients and 6 weeks in 71–90% of patients (5). Although it is not possible to compare these 2 studies, the current study suggests that patients with AD treated with DUP experience fewer relapses and longer times to relapses after discontinuation than those treated with cyclosporine. DUP treatment was restarted, in approximately one-third of patients, a mean of 15.6 weeks after discontinuation. In a comprehensive analysis of the Medicare database, the mean period between discontinuation and re-initiation of DUP was 116 days (6), which is almost the same as that found in the current study. Of the clinical characteristics, only IgE levels seemed to remain stable in the observational period in the exacerbation group (Fig. S2). It was possible that levels of IgE did not change at the initiation of DUP re-treatment in the exacerbation group, because IgE may reflect long-term disease activity (1). Serum levels of DUP decrease gradually after treatment discontinuation, with almost complete elimination of DUP from the body after 8−10 weeks (7, 8). As the mean time of remission in the remission group is 40 weeks, long-term remission seems to be possible after drug elimination. Recently, we found that DUP reduces T helper 2 (Th2) cells and group 2 innate lymphoid (ILC2) cells in patients with AD (9). One of the reasons for long-term remission after discontinuation of DUP may be that the drug changes the Th2/Th17 and ILC2/ILC3 balance, as well as acting as an immunosuppressant.Comparing the baseline clinical characteristics of the exacerbation and remission groups, the patients in the exacerbation group were significantly older than in the remission group in this study. There were no differences in age of AD onset between the 2 groups (Table I), and it was considered that older patients with AD have a longer duration of disease. AD duration may be related to the clinical course after discontinuation of DUP.
This study has some limitations. First, the number of patients was small. Secondly, the data were collected at a single centre in Japan, and the results may differ, depending on race or region. Thirdly, the observation period was short, and patients in the remission group may relapse in the future. Fourthly, we did not have detailed information about concomitant treatment. Because the amount and frequency of the topical applications were determined individually for each patient and oral antihistamines were used when needed according to the Japanese AD guidelines (1), there may be some differences in concomitant treatment between the exacerbation and remission groups.
In conclusion, these results indicate that long-term remission from AD might be achieved after discontinuation of DUP; however, this conclusion is only suggestive due to the limitations of the study, and further studies with more cases are necessary.
ACKNOWLEDGEMENTS
This work was supported by “Hyogo College of Medicine Diversity Grant for Research Promotion” under MEXT Funds for the Development of Human Resources in Science and Technology, “Initiative for Realizing Diversity in the Research Environment (Characteristic-Compatible Type).” This work was supported in part by the Japan Society for the Promotion of Science (YI: KAKENHI 21K08337).
The authors have no conflicts of interest to declare.
REFERENCES
- Katoh N, Ohya Y, Ikeda M, Ebihara T, Katayama I, Saeki H, et al. Japanese guidelines for atopic dermatitis 2020. Allergol Int 2020; 69: 356–369.
- de Wijs LEM, Fujimoto RFT, Andrinopoulou ER, Nijsten T, Hijnen D, Kataoka Y. Dupilumab treatment in patients with atopic dermatitis: a comparative cohort study between the Netherlands and Japan shows a discrepancy in patient-reported outcome measures. Br J Dermatol 2021; 185: 555–562.
- Treister AD, Lio PA. Remittive effect of dupilumab in atopic dermatitis. Dermatol Ther 2018; 31: e12711.
- Worm M, Simpson EL, Thaçi D, Bissonnette R, Lacour JP, Beissert S, et al. Efficacy and safety of multiple dupilumab dose regimens after initial successful treatment in patients with atopic dermatitis a randomized clinical trial. JAMA Dermatol 2020; 156: 131–143.
- Granlund H, Erkko P, Sinisalo M, Reitamo S. Cyclosporin in atopic dermatitis: time to relapse and effect of intermittent therapy. Br J Dermatol 1995; 132: 106–112.
- Silverberg JI, Guttman-Yassky E, Gadkari A, Kuznik A, Mallya UG, Mastey V, et al. Real-world persistence with dupilumab among adults with atopic dermatitis. Ann Allergy Asthma Immunol 2021; 126: 40–45.
- Kovalenko P, Davis JD, Li M, Rippley R, Ardeleanu M, Shumel B, et al. Base and covariate population pharmacokinetic analyses of dupilumab using Phase 3 data. Clin Pharmacol Drug Dev 2020; 9: 756–767.
- Eshtiaghi P, Gooderham MJ. Dupilumab: an evidence-based review of its potential in the treatment of atopic dermatitis. Core Evid 2018; 13: 13–20.
- Imai Y, Kusakabe M, Nagai M, Yasuda K, Yamanishi K. Dupilumab effects on innate lymphoid cell and helper T cell populations in patients with atopic dermatitis. JID Innov 2021; 1: 100003.