Patients with frontal fibrosing alopecia report higher rates of sunscreen use than control subjects. However, it is not known whether the higher use of sunscreens is a cause or a consequence of the alopecia. A greater use of sunscreens should be associated with a lower incidence of signs of actinic damage. The aim of this study is to assess the presence of actinic damage in patients with frontal fibrosing alopecia. A cross-sectional study was carried out on 101 patients with frontal fibrosing alopecia and 40 control subjects. The presence of actinic damage, in the form of solar lentigines, actinic keratoses, and basal and squamous cell carcinomas, was recorded in both groups, together with sunscreen use. Trichoscopy and skin biopsy were performed on patients. Actinic damage was present more frequently in patients with frontal fibrosing alopecia (69.3%) than in control subjects (50%) (p = 0.031). Patients used sunscreens more frequently than did control subjects (83.2% vs 62.5%, p = 0.008). However, the prevalence of trichoscopic inflammatory signs, peripheral alopecia, and inflammatory infiltrate and sebaceous gland involvement in skin biopsy, were similar in patients who used sunscreens and those who did not use them. In conclusion, patients with frontal fibrosing alopecia had greater actinic damage than did control subjects, and this is hypothesized as a reason for the higher use of sunscreens among patients. Thus, use of sunscreens may not be the trigger for frontal fibrosing alopecia that dermatologists have proposed.
Key words: frontal fibrosing alopecia; sunscreen: actinic damage; histopathology; trichoscopy.
Accepted May 23, 2022; Epub ahead of print May 23, 2022
Acta Derm Venereol 2022; 102: adv00757.
DOI: 10.2340/actadv.v102.306
Corr: Trinidad Montero Vílchez, Dermatology Department, University Hospital Virgen de las Nieves, Avenida de Madrid, 15, ES-18012, Granada, Spain. E-mail: tmonterov@gmail.com
SIGNIFICANCE
Patients with frontal fibrosing alopecia have higher rates of sunscreen use than control subjects. For the first time, it has been observed that patients with frontal fibrosing alopecia have greater levels of actinic damage than control subjects without alopecia. The higher use of sunscreens in patients with frontal fibrosing alopecia might be a consequence of this greater actinic damage rather than a cause of the alopecia.
INTRODUCTION
The possible involvement of sunscreen use in the development of frontal fibrosing alopecia (FFA) was proposed in 2016, when some authors found higher use of sunscreens in patients with FFA (48%) compared with a control group (24%) (p < 0.001) (1). The use of sunscreens has increased worldwide in the last 40 years, along with concerns about skin cancer and photoaging. Subsequently, most publications have reported a higher use of sunscreens in patients with FFA (2–4). However, the hypothesis that sunscreen use might be an initial trigger for FFA is highly controversial (5–8) for several reasons:
- Some patients with FFA had not used sunscreens (or, at least, not consciously, since ultraviolet (UV) filters are widely used in moisturizers and make-up) and yet they still developed FFA (9, 10).
- An increasing number of cases of FFA are reported in dark-skinned people, among whom the rates of sunscreen use are generally low (5, 11).
- There could be various reasons why patients with FFA use more sunscreens than control subjects. It may be a new behaviour adopted because of the alopecia, or may reflect higher economic status (which has been observed with respect to patients with FFA) (6, 7, 12).
- The incidence of FFA remains very low compared with the incidence of sunscreen use (5).
Patients with FFA have a higher level of sunscreen use than control subjects (2–4). Sunscreen use prevents actinic damage; that is, the development of pigmented lesions, actinic keratoses and skin cancer (13–15). To our knowledge, actinic damage in patients with FFA has not been assessed. The aim of this study is to evaluate actinic damage in patients with FFA.
MATERIALS AND METHODS
A cross-sectional study was carried out on women with FFA and a control group. Individuals were recruited from the Dermatology Department at the University Hospital San Cecilio and the University Hospital Virgen de las Nieves, Granada, Spain. Inclusion criterion for patients with FFA was the presence of frontal and/or frontotemporal hairline recession, supported by the presence of typical dermoscopic features (loss of follicular openings, perifollicular erythema and follicular hyperkeratosis). Exclusion criteria for patients were cases with no clear diagnosis of FFA and male patients. Patients were under treatment with 5-alpha reductase inhibitors, topical minoxidil and/or topical corticosteroids. Inclusion criteria for the control group were: women aged between 45 and 95 years with no hair disease. Control subjects were people who had consulted the Dermatology Department for other reasons (naevi, seborrhoeic keratosis, etc.). Each participant made 1 visit, at which all the data were recorded. All participants signed an informed consent form and the project was approved by the local ethics committee in Granada.
Demographic information, such as age and ethnic group, was recorded. In patients with FFA, the age of onset of alopecia, the presence of perifollicular erythema and follicular hyperkeratosis, the severity grade and the existence of pruritus and trichodynia were registered. The severity grade was assessed based on the previously described V-grade classification (10), and grouped into mild (I–II) or severe (III–V) FFA. In both groups, the presence of cutaneous signs of actinic damage on the face; that is, solar lentigines, actinic keratoses, and basal cell or squamous cell carcinomas, were recorded, by physical examination and reviewing clinical reports. Regarding sunscreen use, individuals were asked to state “habitual use of sunscreens” (considering habitual use as using sunscreens on at least 5 days per week) over a long period of time (more than 5 years). Skin phototype was evaluated using Fitzpatrick Skin Phototype Classification (16). The presence of peripheral alopecia (in the eyebrows, eyelashes, limbs, axillae and pubis) was also recorded. Furthermore, a 4-mm punch biopsy of skin at the hairline progression was taken from 52 patients with FFA.
Statistical analysis
Student’s t-test was applied to compare the mean values of quantitative variables. Qualitative variables were analysed with the χ2 test. Differences were considered significant at p ≤ 0.05 and nearly significant at p ≤ 0.1. Multivariate logistic regression analyses were performed to explore the variables associated with FFA, sunscreen use and actinic damage. SPSS software (SPSS 20.0, SPSS Inc., Chicago, IL, USA) was used for data analysis.
RESULTS
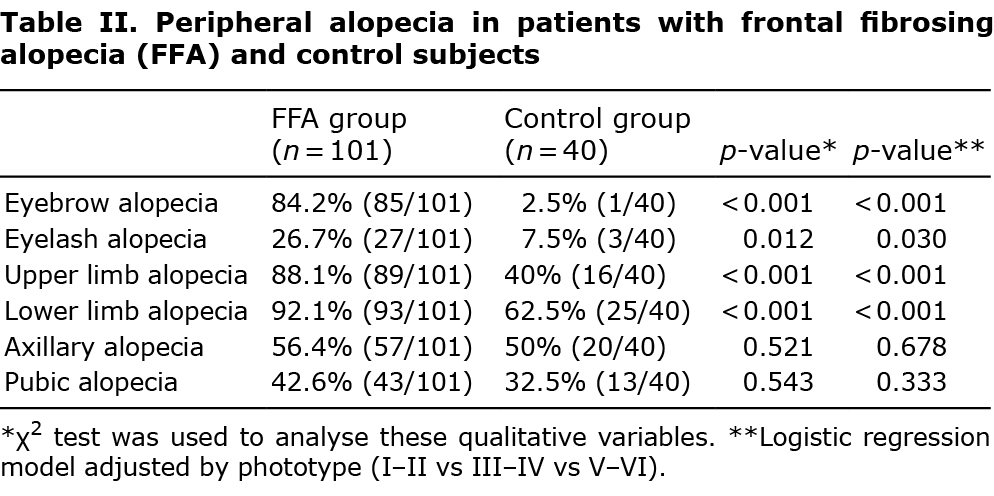
A total of 101 women with FFA and a control group of 40 women were included in the study. Case and control groups were comparable regarding age and ethnic group, although patients with FFA had lower skin phototype than did control subjects (p = 0.012) (Table I). The mean age of onset of alopecia was 58.5 years and the severity of FFA in the sample of patients was: 4% grade I, 42.6% grade II, 33.7% grade III, 10.9% grade IV and 8.9% grade V. Regarding symptoms, 75.2% and 18.8% of patients had pruritus and trichodynia, respectively. Presence of eyebrow, eyelash and limb alopecia was found more frequently in patients with FFA than in control subjects, and this difference was statistically significant (Table II). However, no differences were noted between the groups regarding axillary and pubic alopecia.

Regarding the presence of actinic damage, 69.3% of patients with FFA had actinic damage vs 50% of control subjects (p = 0.031) (Table III). This difference was also observed for the presence of solar lentigines, which were noted in 68.3% of patients vs 47.5% of control subjects (p = 0.021). Regardless of skin phototype, FFA was associated with the presence of actinic damage (p = 0.045) after performing multivariate analysis, especially in the form of solar lentigines (p = 0.029) (Table III).

Concerning the use of sunscreens, 83.2% of patients with FFA used them, compared with 62.5% of control subjects (p = 0.008). Use of sunscreens was not associated with disease severity.
No differences regarding skin phototype or in the presence of peripheral alopecia were found between patients with FFA who used sunscreens and those patients who did not use them. (Table IV).

With reference to trichoscopic signs, perifollicular erythema and follicular hyperkeratosis were noted in 85.1% and 93.1% of patients with FFA, respectively (Table IV). No differences were found regarding the presence of these signs in patients who used sunscreens compared with those who did not use them.
Regarding histopathology, sebaceous gland involvement (reduction or absence) was found in 80.8% of patients, the presence of inflammatory infiltrate was observed in 92.3%, and the presence of inflammatory infiltrate involving follicular infundibulum or isthmus was noted in 51% and 60.8% of patients with FFA, respectively (Table IV). No differences were found regarding the presence of these histopathological signs in sunscreen users compared with non-users. Moreover, a logistic regression model showed that the use of sunscreens (odds ratio (OR) 2.80, p = 0.019) and the presence of actinic damage (OR 2.085, p = 0.084) were independent factors related to FFA (Table V).

DISCUSSION
This study found that sunscreen use and the presence of actinic damage are independent factors related to FFA. Patients with FFA have been shown previously to have higher use of sunscreens compared with control groups (1–5), and this was confirmed in the current study. However, whether this higher use of sunscreens is a cause or a consequence of the alopecia is unknown (6). Patients who consult a dermatologist about a hair problem may have more appearance-related concerns. Indeed, a recent study found a significantly higher rate of facial moisturizer and sunscreen use in both FFA and androgenetic alopecia patients compared with control subjects, suggesting that the use of facial care products may not be truly associated with FFA (17). This behaviour may be due to a reason other than the alopecia, such as higher economic status (6, 12), more frequent visits to a dermatologist, or the presence of another skin alteration. Moreover, daily application of sunscreen to the face has not been associated with worsening disease progression in patients with treated FFA (18). The use of sunscreens in patients with FFA in the current study was not associated with the severity of the disease, in agreement with previous reports (19).
The presence of actinic damage in patients with FFA has not been assessed previously, except for observing the existence of a contrast between the white alopecic band and the photoaged forehead skin. In the current study, a higher prevalence of actinic damage was observed in patients with FFA compared with in control subjects. The most common sign of actinic damage in both groups was the presence of solar lentigines, which were also more common in patients (68.3%) than in control subjects (47.5%). No statistically significant differences were found regarding the presence of actinic keratoses and basal cell carcinoma, despite these being more frequent in patients with FFA, which may be due to the small sample size of those subgroups. The higher prevalence of actinic damage as solar lentigines was also present after carrying out a logistic regression model adjusted for skin phototype. Moreover, it has been shown that patients with FFA have higher rates of rosacea compared with healthy individuals (4, 20); therefore, a more sensitive skin and a lighter skin phototype could also be related to this higher level of actinic damage.
It is not easy to assess individuals’ sunscreen use, either in patients or control subjects, and this may have introduced inaccuracies (5). UV filters are present not only in sunscreens, but also in other skin and haircare products. In a random review of haircare products, 60% of leave-on hair products and 51% of wash-off products contained a chemical sunscreen (21). None of the studies regarding FFA and sunscreens were able to perform subanalyses on sunscreen type, but chemical sunscreens are the most commonly used by the general population and also by patients with FFA (3). The inability to assess specific ingredients in the reported products is also an important limitation in these studies. Recall bias and temporal ambiguity regarding the onset of symptoms in relation to sunscreen use is another relevant limitation. Assessing the exact period of time using sunscreens or moisturizers containing sunscreens is very complicated. However, continuous and prolonged use of sunscreens should be associated with a lower incidence of signs of actinic damage; thus, if patients with FFA had been using sunscreens for a considerable length of time, they should have had a less sun-damaged skin. A likely hypothesis to explain this is that patients with FFA have sun-damaged skin and may have visited a dermatologist for that reason, for which the main medical advice is to avoid sun exposure and to use sunscreens; or they may have started using sunscreens due to the development of solar lentigines.
Regarding trichoscopic signs in FFA, perifollicular erythema has been considered to be a marker of FFA activity (22), and many patients with a receding hairline have persistent inflammatory signs (perifollicular erythema and follicular hyperkeratosis). However, there is increasing recognition that these inflammatory signs may persist in patients despite there being no progression in hairline recession (12, 23, 24), and others may have hair loss progression without inflammatory signs (24). No differences were found regarding the presence of perifollicular erythema or follicular hyperkeratosis in patients with FFA who used sunscreens compared with those who did not use sunscreens. Considering those trichoscopic signs as diagnostic clues and, to some extent, related to disease activity, if sunscreen use was related to the development of FFA, differences would be expected between sunscreen-users and non-users.
Concerning histopathological signs, the atrophy or loss of sebaceous glands is considered an early sign of FFA, along with the inflammatory infiltrate involving hair, but without perifollicular fibrosis (which is a more advanced sign) (25–27). No differences were observed between patients with FFA who used sunscreens compared with those who did not use them in relation to sebaceous gland involvement, the presence of inflammatory infiltrate or the presence of inflammatory infiltrate involving infundibulum or isthmus. Therefore, the use of sunscreens does not appear to be related to a higher prevalence of histopathological alterations.
Alopecia at other body sites has also been noted in patients with FFA, especially on the eyebrows (63–83%) (10, 23, 28), but also on eyelashes (3–14%) (10, 12), limbs (17–24%) (10, 23) axillae (21%) and pubis (18%) (10). Reduction in peripheral hair (mostly on the limbs, axillae and pubis) is a common finding in healthy women after menopause. Moreover, no clear association between alopecia and sunscreen use on the rest of the body has been reported (5). In the current cohort, the presence of eyebrow, eyelash and limb alopecia was more prevalent in women with FFA than in control individuals and this difference reached statistical significance (but not for axillary and pubic alopecia). Nevertheless, no differences were found in relation to the use of sunscreens and the presence of peripheral alopecia in patients with FFA.
Study limitation
The main limitation of this study is the presence of recall bias regarding sunscreens use and the type of sunscreen (physical/chemical). This bias is similar in patients and control subjects, as they were asked the same question about the use of sunscreens; hence, it is a non-differential bias. There could also be recall bias regarding any previous history of skin tumours among the few participants who did not have any medical history at the hospital. A further limitation may be the fact that patients were under treatment for FFA.
Conclusion
Patients with FFA had higher levels of actinic damage than control subjects, which could be a reason for their higher use of sunscreens. Trichoscopic inflammatory signs, either the presence of sebaceous gland damage or inflammatory infiltrate, were not more frequent in patients with FFA who used sunscreens than in those patients who did not use them. Moreover, no differences were found regarding the presence of peripheral alopecia in patients with FFA who used sunscreens compared with those who did not use them. Therefore, sunscreen use may not be the trigger for FFA that some dermatologists have suggested. Further research is required to confirm this hypothesis, and to determine whether there are other possible triggers for FFA, as suggested by the increased prevalence of FFA.
ACKNOWLEDGEMENTS
This article is part of the PhD thesis of María Librada Porriño-Bustamante.
The authors have no conflicts of interest to declare.
REFERENCES
- Aldoori N, Dobson K, Holden CR, McDonagh AJ, Harries M, Messenger AG. Frontal fibrosing alopecia: possible association with leave-on facial skin care products and sunscreens; a questionnaire study. Br J Dermatol 2016; 175: 762–767.
- Debroy Kidambi A, Dobson K, Holmes S, Carauna D, Del Marmol V, Vujovic A, et al. Frontal fibrosing alopecia in men: an association with facial moisturizers and sunscreens. Br J Dermatol 2017; 177: 260–261.
- Moreno-Arrones OM, Saceda-Corralo D, Rodrigues-Barata AR, Castellanos-González M, Fernández-Pugnaire MA, Grimalt R, et al. Risk factors associated with frontal fibrosing alopecia: a multicentre case-control study. Clin Exp Dermatol 2019; 44: 404–410.
- Porriño-Bustamante ML, Fernández-Pugnaire MA, Arias-Santiago S. A cross-sectional study of rosacea and risk factors in women with frontal fibrosing alopecia. Acta Derm Venereol 2019; 99: 1099–1104.
- Robinson G, McMichael A, Wang SQ, Lim HW. Sunscreen and frontal fibrosing alopecia: a review. J Am Acad Dermatol 2020; 82: 723–728.
- Donati A. Frontal fibrosing alopecia and sunscreens: cause or consequence? Br J Dermatol 2016; 175: 675–676.
- Dhana A, Gumedze F, Khumalo NP. Regarding “Frontal fibrosing alopecia: possible association with leave-on facial skincare products and sunscreens; a questionnaire study”. Br J Dermatol 2017; 176: 836–837.
- Porriño-Bustamante ML, Fernández-Pugnaire MA, Arias-Santiago S. Frontal fibrosing alopecia: a review. J Clin Med 2021; 10: 1805.
- Ormaechea-Pérez N, López-Pestaña A, Zubizarreta-Salvador J, Jaka-Moreno A, Panés-Rodríguez A, Tuneu-Valls A. Frontal fibrosing alopecia in men: presentations in 12 cases and a review of the literature. Actas Dermosifiliogr 2016; 107: 836–844.
- Vañó-Galván S, Molina-Ruiz AM, Serrano-Falcón C, Arias-Santiago S, Rodrigues-Barata AR, Garnacho-Saucedo G, et al. Frontal fibrosing alopecia: a multicenter review of 355 patients. J Am Acad Dermatol 2014; 70: 670–678.
- Dlova NC, Jordaan HF, Skenjane A, Khoza N, Tosti A. Frontal fibrosing alopecia: a clinical review of 20 black patients from South Africa. Br J Dermatol 2013; 169: 939–941.
- MacDonald A, Clark C, Holmes S. Frontal fibrosing alopecia: a review of 60 cases. J Am Acad Dermatol 2012; 67: 955–961.
- Naylor MF, Farmer KC. The case for sunscreens. A review of their use in preventing actinic damage and neoplasia. Arch Dermatol 1997; 133: 1146–1154.
- Hölzle E. Pigmented lesions as a sign of photodamage. Br J Dermatol 1992; 127: 48–50.
- Young AR, Claveau J, Rossi AB. Ultraviolet radiation and the skin: photobiology and sunscreen photoprotection. J Am Acad Dermatol 2017; 76: S100–S109.
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol 1988; 124: 869–871.
- Leecharoen W, Thanomkitti K, Thuangtong R, Varothai S, Triwongwaranat D, Jiamton S, et al. Use of facial care products and frontal fibrosing alopecia: coincidence or true association? J Dermatol 2021; 48: 1557–1563.
- Imhof RL, Larkin SC, Cantwell HM, Torgerson RR, Tolkachjov SN. The association of frontal fibrosing alopecia with skin and hair care products: a survey-based case series of 56 patients seen at Mayo Clinic. J Am Acad Dermatol 2020; 84: 532–534.
- Moreno-Arrones OM, Saceda-Corralo D, Rodrigues-Barata AR, Castellanos-González M, Fernández-Pugnaire MA, Grimalt R, et al. Factors influencing frontal fibrosing alopecia severity: a multicentre cross-sectional study. J Eur Acad Dermatol Venereol 2019; 33: e315–e316.
- Pindado-Ortega C, Saceda-Corralo D, Buendía-Castaño D, Fernández-González P, Monero-Arrones Ó, Fonda-Pascual P, et al. Frontal fibrosing alopecia and cutaneous comorbidities: a potential relationship with rosacea. J Am Acad Dermatol 2018; 78: 596–597.
- Callander J, Frost J, Stone N. Ultraviolet filters in hair-care products: a possible link with frontal fibrosing alopecia and lichen planopilaris. Clin Exp Dermatol 2018; 43: 69–70.
- Toledo-Pastrana T, Hernández MJ, Camacho Martínez FM. Perifollicular erythema as a trichoscopy sign of progression in frontal fibrosing alopecia. Int J Trichology 2013; 5: 151–153.
- Tan KT, Messenger AG. Frontal fibrosing alopecia: clinical presentations and prognosis. Br J Dermatol 2009; 160: 75–79.
- Saceda-Corralo D, Pindado-Ortega C, Moreno-Arrones OM, Ortega-Quijano D, Fernández-Nieto D, Jiménez-Cauhe J, et al. Association of inflammation with progression of hair loss in women with frontal fibrosing alopecia. JAMA Dermatol 2020; 156: 700–702.
- Pirmez R, Duque-Estrada B, Abraham LS, Pinto GM, de Farias DC, Kelly Y, et al. It’s not all traction: the pseudo ‘fringe sign’ in frontal fibrosing alopecia. Br J Dermatol 2015; 173: 1336–1338.
- Katoulis AC, Damaskou V, Diamanti K, Pouliakis A, Mortaki D, Zacharatou A, et al. Eyebrow involvement in frontal fibrosing alopecia: a clinicopathologic cohort study for the reversibility of hair loss. J Am Acad Dermatol 2020; 82: 755–757.
- Miteva M, Sabiq S. A new histologic pattern in 6 biopsies from early frontal fibrosing alopecia. Am J Dermatopathol 2019; 41: 118–121.
- Moreno-Ramírez D, Ferrándiz L, Camacho FM. [Diagnostic and therapeutic assessment of frontal fibrosing alopecia]. Actas Dermosifiliogr 2007; 98: 594–602.