ORIGINAL REPORT
Validation of the Recap of Atopic Eczema (RECAP) Measurement Instrument for Eczema Control in Adult Patients in an Asian Clinical Setting
Yik Weng YEW1,2, Crystal Zhen Yu PHUAN1, Xiahong ZHAO1, Laura HOWELLS3 and Christian J. APFELBACHER4,5
1National Skin Centre, Singapore, 2Lee Kong Chian School of Medicine, Nanyang Technological University Singapore, 3University of Nottingham Centre for Evidence Based Dermatology, Nottingham, UK, 4Institute of Social Medicine and Health Systems Research, Otto von Guericke University Magdeburg, Magdeburg, Germany, and 5Family Medicine and Primary Care, Lee Kong Chian School of Medicine, Nanyang Technological University Singapore, Singapore
Recap of atopic eczema (RECAP) is a self-reported 7-item questionnaire recommended by the Harmonising Outcome Measures in Eczema initiative to measure eczema control. As RECAP has not been validated in a real-world clinical population in Asia, RECAP was investigated as a measure of eczema control in Singapore. Patients with atopic eczema at the National Skin Centre from July 2019 to January 2020 were included for analysis. Both patient- and physician-reported outcome measures were available for correlation analyses. Correlation analysis was also performed to investigate construct validity, and floor or ceiling effects of RECAP. A total of 260 atopic eczema patients aged between 15 and 87 years were recruited. There were minimal floor and ceiling effects for RECAP scores. There were strong, significant correlations of RECAP with POEM (r = 0.84, p < 0.001) and DLQI (r = 0.81, p < 0.001). Correlation with SCORAD was moderate (r = 0.60, p < 0.001). Correlations remained similar after age, gender, and ethnicity adjustments. Discriminative validity was demonstrated by a significant linear trend of increasing RECAP scores with increasing eczema severity. RECAP demonstrates good discriminative and construct validity evidenced by strong correlations with symptoms and quality of life and moderate correlations with eczema signs. RECAP is useful to measure eczema control in Singapore.
Key words: atopic eczema; outcomes; measurement.
SIGNIFICANCE
Atopic eczema is a common skin condition with a huge disease burden. It is important for managing clinicians to accurately assess disease control in response to treatments and interventions. Various outcome measures are available and recommended for measuring different facets of eczema control. Recap of atopic eczema (RECAP) is a measure intended to capture the various aspects of disease control as an overall global measurement instrument. It has been assessed to be relevant and useful in various European clinical populations. But it is also important to understand if this is similarly relevant among other patients with culturally diverse backgrounds.
Citation: Acta Derm Venereol 2024; 104: adv32323. DOI https://doi.org/10.2340/actadv.v104.32323.
Copyright: © 2024 The Author(s). Published by MJS Publishing, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/).
Submitted: Nov 28, 2024; Accepted: Mar 22, 2024; Published: May 13, 2024
Corr: Yik Weng Yew, National Skin Centre, 1 Mandalay Road, Singapore 308205. E-mail: yikweng.yew@ntu.edu.sg
Competing interests and funding: CJA is a member of the executive committee of the Harmonising Outcomes Measures for Eczema (HOME) initiative and lead of the quality of life working group within HOME. He was involved in the development of RECAP, He has received honorariums and institutional funding from Dr Wolff GmbH, and honorariums from Sanofi Genzyme. LH is a member of the HOME executive committee and was involved in the development of RECAP. She has received consultancy fees from the University of Oxford on an educational grant funded by Pfizer, unrelated to the submitted work. She is employed at the research centre where the Patient Oriented Eczema Measure (POEM) was developed. The University of Nottingham owns copyright to license the Patient Oriented Eczema Measure – chargeable for commercial users. The remaining authors declare that they have no conflicts of interest.
INTRODUCTION
A topic eczema (AE) is one of the most prevalent chronic inflammatory skin diseases globally, characterized mainly by pruritus (1, 2). Its pathophysiology is complex and includes multiple factors including epithelial barrier dysfunction, immune dysregulation, and psycho-neurogenic inflammation (3–6). The burden of AE is closely correlated with disease severity, with significant economic burden for both the affected individual and for society with marked impairment in quality of life (QoL) (7–9).
Numerous outcome measures have been developed to accurately measure various disease outcomes and domains for AE (10). This includes physician-reported measures such as the Eczema Area and Severity Index (EASI) and SCORing Atopic Dermatitis (SCORAD), patient-reported outcomes such as the Patient-Oriented Eczema Measure (POEM), as well as quality of life measures such as the Dermatology Life Quality Index (DLQI). There are both strengths and weaknesses for each outcome measure. For example, while EASI and POEM are comprehensive, EASI has unclear interpretability with prolonged administration time while POEM focuses mainly on the disease symptoms as experienced by patients (11, 12). SCORAD has good content and construct validity but, similar to EASI, has a prolonged time of administration (13, 14). In addition, these measures are insufficient to accurately reflect the global control of the AE. To address this problem, the Harmonising Outcome Measures for Eczema (HOME) initiative included long-term eczema control as one of the 4 domains deemed important to measure in all trials of eczema. Together with the domains physician-reported signs, patient-reported symptoms, and quality of life, these formed a core outcome set to be used for all clinical trials (15, 16). It was only during HOME V that the addition of a patient-reported global instrument to capture eczema control was recommended (17, 18). The HOME VII meeting in 2019 recommended the use of the Atopic Dermatitis Control Test (ADCT) or RECAP as measures for long-term control as part of the core set of outcomes to be reported in all eczema trials (16, 17). RECAP was developed and validated in English, but currently other language versions of RECAP such as German, Dutch, and Spanish versions were also considered comprehensible, and adults, as well as parents of affected children (19–21).
Both RECAP and ADCT were developed independently to be used as a patient- or caregiver-reported instrument to capture an individual’s experience of eczema control (22, 23). They are similar, with multiple-item scales and a recall period of 1 week. Both questionnaires cover similar domains with similar response options scored 0–4, although RECAP has an additional question to differentiate itch from intense itch. RECAP, which comprises 7 questions, has recently been adopted as a patient global assessment of eczema control at National Skin Centre (NSC), a tertiary dermatology centre in Singapore. Initial testing of score distribution and construct validity of RECAP in a population with eczema in the UK suggests good measurement properties to measure eczema control in clinical trials and in routine practice (15). As RECAP has not been validated in an Asian clinical setting, we investigated the acceptability, construct validity, and reliability of RECAP as a measure of eczema control in our clinical setting with Asian adult eczema patients. We also aimed to evaluate feedback of the instrument using a survey feedback form in a small subset of the participants (n = 13).
MATERIALS AND METHODS
Patient population
Singaporean patients diagnosed with AE by dermatologists, attending the adult eczema clinic at NSC from 1 July 2019 to 31 January 2020 were included and self-completed POEM and DLQI in addition to RECAP in the English language as part of their routine clinical assessment were analysed. In addition, physician outcome measures such as SCORAD and EASI scores were available for only a subset of patients as these scores depended on the respective attending physicians for completion in a busy clinical practice. These data were collected at the initial visit and the follow-up visit, ranging from 2 to 3 months after the first visit. The diagnosis of AE was confirmed by certified dermatologists based on medical history, clinical features, and, in a small subset, skin biopsy. Patients who were unable to understand English were excluded from this study. Anonymized and de-identified data and information were collected as part of a routine clinical audit and granted exemption by the local institutional ethics board.
Validity assessment
RECAP is based on a formative model, meaning that the items are not expected to correlate with each other. Rather, they represent unique aspects that together form eczema control (24). Construct validity was assessed by hypotheses testing. Correlation analysis was performed to assess the relationship between POEM and RECAP and Spearman correlation coefficients were reported, with r≤0.3 as poor (+), 0.3<r<0.7 as moderate (++) and r≥0.7 as strong (+++) (25). Instruments measuring a similar construct would be expected to be strongly correlated. As RECAP is a patient-reported instrument that also incorporates components of disease severity as well as quality of life, additional instruments included in the correlation analysis were SCORAD, EASI, and DLQI. A priori hypotheses concerning the correlation between RECAP and the other instruments were made and are listed in Table I. Assessment for discriminant validity was performed by calculating mean RECAP scores across previously published severity bandings of POEM, with the following hypothesis: there would be higher mean RECAP scores with worsening disease severity bandings. This would suggest that the RECAP instrument is able to differentiate AE patients with different disease severity. Similar analyses were performed by calculating mean RECAP scores across SCORAD and DLQI severity bandings.
| A priori hypotheses | Measures | Correlation |
| RECAP | POEM | ++ (moderate) |
| SCORAD | + (poor) | |
| EASI | + (poor) | |
| DLQI | ++ (moderate) | |
| Spearman correlation coefficients are classified, with r≤0.3 being poor (+), 0.3<r<0.7 moderate (++) and r≥0.7 strong (+++) (25). Instruments measuring a similar construct would be expected to be strongly correlated. | ||
Feasibility was performed by an assessment of the distribution of scores on a histogram. Floor and ceiling analysis was performed for each RECAP item and its overall RECAP score. If 15% of the data was observed at the minimum or maximum points of each item, then a floor or ceiling effect would exist (26, 27).
Feedback survey/acceptability
Missing data analysis and user feedback analysis were conducted to access the acceptability. A subset of participants (n = 13) were asked to comment on the overall experience, understanding, relevance, and length of time needed to complete the survey based on an open-ended structured survey. They were also asked to suggest whether any important questions were missing from the RECAP questionnaire. A total of 5 specific feedback questions and a sixth question on “Any other comments or feedback” were used to assess the RECAP questionnaire (see Table VI).
Statistical analysis
Categorical variables were summarized using counts and percentages. Continuous variables were summarized using mean with standard deviation (SD) or median with range. Significance was assessed at a significant level of 0.05. Data analysis was done using SPSS 25.0 for Windows (IBM Corp, Armonk, NY, USA).
RESULTS
Patient population
A total of 260 patients with AE completed RECAP within the observation period. A subset of 43 patients completed RECAP at a subsequent visit, 2 to 3 months later, giving a total of 303 responses for analysis. Their age ranged from 15 to 87 years with a median age of 25.8 years. More than 76% of the participants were male. The majority (87.1%) of participants were Chinese, followed by Malays (8.3%), and Indians (2.0%) (Table II). The majority of patients included in the study had moderate to severe disease severity, with 61.0% (61 out of 103) patients having a moderate to severe SCORAD score and 80.2% (207 out of 258) patients having a moderate to severe POEM score. While almost all patients completed the patient-reported outcome measures such as POEM (n = 258) and DLQI (n = 253), only some patients had their SCORAD (n = 103) and EASI (n = 17) scores completed by their attending physicians.
Validity assessment
RECAP showed good convergent validity with POEM, DLQI, and SCORAD. The correlations of RECAP with POEM, DLQI, and SCORAD were moderate to strong (r(299)=0.84, p < 0.001; r(297)=0.81, p < 0.001, and r(103)=0.60, p < 0.001 respectively) (Fig. 1). The relationship remained significant, and the partial Spearman correlation remained strong after adjusting for age, gender, and ethnicity. With a priori hypothesized correlation values as provided in Table I, convergent validity was clearly demonstrated for RECAP when compared against POEM and DLQI. In fact, the correlations of RECAP with POEM and DLQI were strong (r ≥ 0.7). Linear regression also showed that RECAP total score had significant positive correlations with POEM and DLQI, which accounted for 70.4% and 64.6% of the variance observed in the adjusted regression model respectively (p < 0.0001) (Table III). The correlation of RECAP and SCORAD was moderate at r = 0.60 and was better than the a priori hypothesized correlation. The correlation with EASI was moderate and not significant (r(17)=0.42, p = 0.096) but this was better than the a priori hypothesized value of r < 0.3.
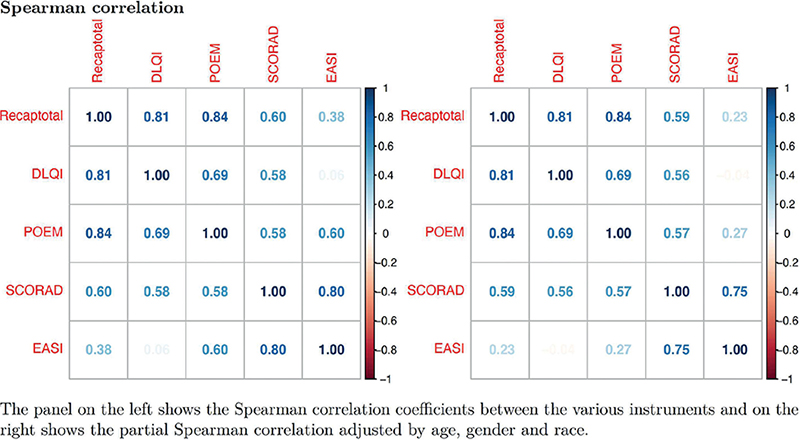
Fig. 1. Heatmap Spearman correlation plots of RECAP against other instruments.
The RECAP total scores in this study cohort ranged from 0 to 28 and followed a normal distribution closely (Fig. 2). There were no floor and ceiling effects for RECAP total score at 0.4% and 3.1% respectively at baseline and 0% at a subsequent visit (Table IV). The data demonstrated a significant linear trend of RECAP scores with increasing severity of eczema by both POEM and SCORAD (p < 0.001 and p < 0.001). There were higher mean RECAP scores among patients with more severe eczema, across the increasing severity banding of POEM, SCORAD, and DLQI (Table V). This supported the hypothesis that there was discriminative validity.
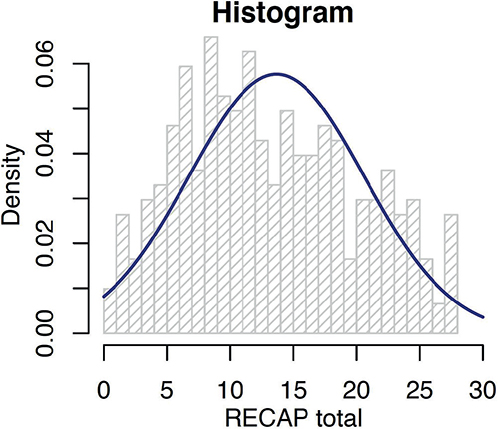
Fig. 2. Distribution of RECAP total score.
Qualitative feedback/acceptability
RECAP demonstrated good user feedback with missing data for individual items being 0.4% at baseline and 0% at follow-up visit. Survey feedback was obtained regarding the RECAP questionnaire among a subset of participants (n = 13) during the early stages of implementation of RECAP (Table VI). Overall feedback was good. With regard to areas for improvement, patients suggested that RECAP should address the efficacy of current treatments, location of itch, and use a wider numerical scale.
DISCUSSION
This is the first study that examines the validity of RECAP in an Asian adult clinical setting of adequate sample size with comparison with objective physician-reported measures such as SCORAD and EASI. The study also examined the reliability as well as acceptability using a feedback survey. There was evidence for good convergent and discriminant validity. Total RECAP scores were well normally distributed across the mild to severe range of AE in our study cohort. The scores were able to differentiate between patients in milder and more severe AE bandings. There were also no floor or ceiling effects for the overall total RECAP scores. These findings were consistent with the development and initial testing of the RECAP instrument as well as other validation studies in Europe, with 1 study including patients from the outpatient dermatology department in Bristol, UK (19–22, 28, 29). Our study demonstrated strong correlations of RECAP with other eczema outcome measures. The correlation of RECAP with POEM was 0.84 in our study and this is very similar to the correlations between RECAP and POEM of the other 2 validation studies of 0.83 and 0.75 respectively (22, 29). Besides its correlations with POEM, our study has also provided additional evidence of strong correlations with DLQI and moderate correlations with SCORAD and EASI. This is consistent with our original hypothesis that RECAP is a good instrument to measure eczema control in adult patients.
Our study also examined the feedback on individual items of the instrument in a feedback survey. During the feedback survey, the majority of our patients felt that the RECAP was relevant, easy to complete, and recognizes the physical and psychological challenges that afflict patients with eczema. Furthermore, they also felt that RECAP was comprehensive and encompassed all the important aspects which their physicians should know about their condition. However, there was also some feedback and suggestions.
One patient commented that RECAP had not included the efficacy of current treatment in its measurement. The concept of including efficacy of current treatments was considered during the original development work (22). It was, however, noted to be challenging during development to create a universal question regarding treatment. We acknowledge that this could therefore potentially be a missing element in the RECAP measure. Another patient provided the feedback that RECAP did not include the location of itch in eczema patients. However, this might not be part of the primary aim of RECAP, which was to capture the experience of eczema control. Accompanying documentation on the location of itch in the medical records alongside RECAP could potentially address this limitation. Finally, a patient has suggested that the response scale could potentially be widened beyond 0 to 4 to provide better granularity. While this is possible, having more points on the scale would make future comparisons with the original validation studies less comparable and might be less desirable. Analysis of responses in our cohort has also not revealed obvious ceiling or floor effects of the scale.
Eczema control is multi-dimensional, with its success being influenced by changes in disease activity, treatment, and management of the condition on top of psychological, social, and physical functioning (30). An international survey found that 75% of patients and caregivers rated effective control of their eczema as the most important factor contributing to improvement in their QoL (31). In addition to QoL, there is a significant economic burden as well (32). RECAP’s correlations with other measures suggest that RECAP captures dimensions that these other tools also measure. It was indeed specifically designed to combine the multifaceted aspects that impact perception of eczema control into an overall global measurement instrument. As recommended by HOME’s core outcomes set, it is intended for use in routine care and clinical trials, and was designed to maximize comprehensiveness, comprehensibility, and relevance to patients and caregivers while producing a feasible tool to evaluate eczema control (33). A systematic review based on the COnsensus-based Standards for the selection of health Measurement INstruments (COSMIN) assessment process has looked at studies assessing the measurement properties of instruments to measure eczema control and concluded that both RECAP and ADCT have sufficient evidence to support them being recommended for inclusion in the core outcomes set for eczema (34). The content validity of RECAP has also been assessed in various studies (19–21, 28, 29). The self-reported version of RECAP has been reported to be appropriate for use from the age of 12 years (20).
Having a standardized, valid instrument for global control of eczema in clinical trials is important, as this will allow comparison between various treatment agents in different clinical trials, with outcome measures being clinically important and meaningful to patients. In our experience of using RECAP at NSC, RECAP is also suitable for use in a clinical setting as it is easy to use, time efficient, and is a capture of global measure. Furthermore, this also provides the opportunity to explore the potential use of self-administered instruments at home, which will allow remote feedback to physicians. In the era of the COVID-19 pandemic where tele-dermatology plays a greater role in clinical care, having a global measure that can be self-administered will enable physicians to monitor their patients’ progress closely to ensure good AE control, possibly even remotely.
The main limitation of this study is that the majority of our participants were Chinese, with a fair representation of Malays and Indians. This could potentially affect our interpretation and generalizability in minority ethnic groups. With a smaller representation in minor ethnic groups, we were therefore not able to investigate the role of cultural perceptions in eczema control. A more diverse participant pool for future studies beyond East Asians would provide more comprehensive demographic representation in Asia. The majority of our participants were male. As disease prevalence and symptoms could vary between genders, a more balanced gender distribution in future studies could further strengthen the study’s validity. Only participants who could understand English were recruited, as the English-language versions of the different questionnaire measures were used. However, given the relatively common usage of English as a working language in Singapore, its impact on generalizability might be kept to a minimum. Paediatric patients were excluded from the study. Also, while there was a good range of disease severity among study participants, more than half of the participants had moderate to severe eczema, mainly because the study was conducted in the eczema specialty clinic. There were also missing SCORAD and EASI scores for many patients, as not all dermatologists completed their objective scores. These might have affected the correlations and statistical significance given the small sample size. However, all patients were assessed and examined by dermatologists. While patients provided a qualitative feedback concerning RECAP, this is limited to a small subset of patients given that this was implemented during the initial day of RECAP implementation in the clinic.
To our knowledge, this is the first study to examine the validity of RECAP among adult Asian eczema patients of different ethnicities in a clinical setting. It is important to test RECAP in various ethnic populations. RECAP has been proposed as a measure for long-term control for eczema trials. It is therefore important that results in clinical trials would be interpretable in various population settings for wider adoption. The cultural differences could affect the perception of disease severity and quality of life. We hypothesize that Asian patients could potentially underreport their disease severity given the cultural background. Notably, this is also among the first studies to examine its correlation with other outcome measures and especially physician-reported outcome measures such as SCORAD and EASI. RECAP shows good construct validity evidenced by strong correlations with symptoms and QoL and moderate correlations with eczema signs. Our study confirms that RECAP is useful and appropriate as an instrument to measure eczema control in our Asian clinical setting. It should be incorporated as a regular disease control measure alongside other patient- and physician-reported measures in our routine clinical care of eczema patients. Its ease of use also provided a form of global eczema control monitoring in situations where physician outcome scores such as SCORAD or EASI may not be readily available. However, future studies should evaluate how RECAP performs in milder disease as well as among young people and their caregivers in our Asian clinical setting. Further validation studies in various Asian populations of different cultures and native languages would also be required. Other translated language versions of RECAP such as a Chinese-language version could also be further validated in different Asian settings.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the clinical audit team and Research Division of the National Skin Centre as well as the patients who have participated in this anonymous audit questionnaire project.
IRB approval status: Anonymized and de-identified data and information were collected as part of a routine clinical audit. The study fulfilled the criteria for exemption by the local institutional ethics board, DSRB.
REFERENCES
- McNally N, Phillips D. Geographical epidemiology of atopic dermatitis. Cambridge: Cambridge University Press; 2000.
- Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, et al. Revised nomenclature for allergy for global use: Report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol 2004; 113: 832–836.
- Eyerich K, Eyerich S. Immune response patterns in non-communicable inflammatory skin diseases. J Eur Acad Dermatol Venereol 2018; 32: 692–703.
- Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet 2006; 38: 441–446.
- Weidinger S, Beck LA, Bieber T, Kabashima K, Irvine AD. Atopic dermatitis. Nat Rev Dis Primers 2018; 4: 1.
- Weisshaar E, Diepgen TL, Bruckner T, Fartasch M, Kupfer J, Lob-Corzilius T, et al. Itch intensity evaluated in the German Atopic Dermatitis Intervention Study (GADIS): correlations with quality of life, coping behaviour and SCORAD severity in 823 children. Acta Derm Venereol 2008; 8: 234–239.
- Ring J, Zink A, Arents BWM, Seitz IA, Mensing U, Schielein MC, et al. Atopic eczema: burden of disease and individual suffering – results from a large EU study in adults. J Eur Acad Dermatol Venereol 2019; 33: 1331–1340.
- Blome C, Radtke MA, Eissing L, Augustin M. Quality of life in patients with atopic dermatitis: disease burden, measurement, and treatment benefit. Am J Clin Dermatol 2016; 17: 163–169.
- Silverberg JI, Gelfand JM, Margolis DJ, Boguniewicz M, Fonacier L, Grayson MH, et al. Patient burden and quality of life in atopic dermatitis in US adults: a population-based cross-sectional study. Ann Allergy Asthma Immunol 2018; 121: 340–347.
- Burke LB, Kennedy DL, Miskala PH, Papadopoulos EJ, Trentacosti AM. The use of patient-reported outcome measures in the evaluation of medical products for regulatory approval. Clin Pharmacol Ther 2008; 84: 281–283.
- Bozek A, Reich A. Assessment of intra- and inter-rater reliability of three methods for measuring atopic dermatitis severity: EASI, objective SCORAD, and IGA. Dermatology 2017; 233: 16–22.
- Charman CR, Venn AJ, Williams HC. The patient-oriented eczema measure: development and initial validation of a new tool for measuring atopic eczema severity from the patients’ perspective. Arch Dermatol 2004; 140: 1513–1519.
- Schmitt J, Langan S, Williams HC, European Dermato-Epidemiology Network. What are the best outcome measurements for atopic eczema? A systematic review. J Allergy Clin Immunol 2007; 120: 1389–1398.
- Schmitt J, Langan S, Deckert S, Svensson A, von Kobyletzki L, Thomas K, et al. Assessment of clinical signs of atopic dermatitis: a systematic review and recommendation. J Allergy Clin Immunol 2013; 132: 1337–1347.
- Schmitt J, Spuls P, Boers M, Thomas K, Chalmers J, Roekevisch E, et al. Towards global consensus on outcome measures for atopic eczema research: results of the HOME II meeting. Allergy 2012; 67: 1111–1117.
- Thomas KS, Apfelbacher CA, Chalmers JR, Simpson E, Spuls PI, Gerbens LAA, et al. Recommended core outcome instruments for health-related quality of life, long-term control and itch intensity in atopic eczema trials: results of the HOME VII consensus meeting. Br J Dermatol 2021; 185: 139–146.
- Chalmers JR, Thomas KS, Apfelbacher C, Williams HC, Prinsen CA, Spuls PI, et al. Report from the fifth international consensus meeting to harmonize core outcome measures for atopic eczema/dermatitis clinical trials (HOME initiative). Br J Dermatol 2018; 178: e332–e341.
- Leshem YA, Chalmers JR, Apfelbacher C, Katoh N, Gerbens LAA, Schmitt J, et al. Measuring atopic eczema control and itch intensity in clinical practice: a consensus statement from the Harmonising Outcome Measures for Eczema in Clinical Practice (HOME-CP) Initiative. JAMA Dermatol 2022; 158: 1429–1435.
- Gabes M, Tischer C, Herrmann A, Howells L, Apfelbacher C. The German RECAP questionnaire: linguistic validation and cognitive debriefing in German adults with self-reported atopic eczema and parents of affected children. J Patient Rep Outcomes 2021; 5: 13.
- Gabes M, Ragamin A, Baker A, Kann G, Donhauser T, Gabes D, et al. Content validity of the Recap of atopic eczema (RECAP) instrument in Dutch, English and German to measure eczema control in young people with atopic eczema: a cognitive interview study. Br J Dermatol 2022; 187: 919–926.
- Onteniente-Gomis MM, Ortiz-Romero PL, Tous Romero F, Salamanca Castro AB, Ortiz de Frutos FJ. Spanish Version of the RECAP questionnaire to assess control of atopic eczema: translation, cultural adaptation, validation, and correlations with other patient-reported outcome measures. Actas Dermosifiliogr 2023; 114: 488–493.
- Howells LM, Chalmers JR, Gran S, Ahmed A, Apfelbacher C, Burton T, et al. Development and initial testing of a new instrument to measure the experience of eczema control in adults and children: Recap of atopic eczema (RECAP). Br J Dermatol 2020; 183: 524–536.
- Simpson E, Eckert L, Gadkari A, Mallya UG, Yang M, Nelson L, et al. Validation of the Atopic Dermatitis Control Tool (ADCT(c)) using a longitudinal survey of biologic-treated patients with atopic dermatitis. BMC Dermatol 2019; 19(1): 15.
- De Vet HC TC, Mokkink LB, Knol DL. Measurement in medicine: a practical guide. New York: Cambridge University Press; 2011.
- Dancey CP, Reidy J. Statistics without maths for psychology. Harlow, UK: Pearson/Prentice Hall; 2007.
- Terwee CB, Bot SD, de Boer MR, van der Windt DA, Knol DL, Dekker J, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol 2007; 60: 34–42.
- McHorney CA, Tarlov AR. Individual-patient monitoring in clinical practice: are available health status surveys adequate? Qual Life Res 1995; 4: 293–307.
- Bhanot A, Peters TJ, Ridd MJ. Assessing the validity, responsiveness and reliability of the Recap measure of eczema control. Br J Dermatol 2021; 184: 955–957.
- Bhanot A, Vincent R, Peters TJ, Ridd MJ. Validation of the RECap of AtoPic eczema measure of eczema control for use in dermatology clinics. Clin Exp Dermatol 2022; 47: 440–442.
- Howells LM, Chalmers JR, Cowdell F, Ratib S, Santer M, Thomas KS. ‘When it goes back to my normal I suppose’: a qualitative study using online focus groups to explore perceptions of ‘control’ among people with eczema and parents of children with eczema in the UK. BMJ Open 2017; 7: e017731.
- Zuberbier T, Orlow SJ, Paller AS, Taieb A, Allen R, Hernanz-Hermosa JM, et al. Patient perspectives on the management of atopic dermatitis. J Allergy Clin Immunol 2006; 118: 226–232.
- Zink AGS, Arents B, Fink-Wagner A, Seitz IA, Mensing U, Wettemann N, et al. Out-of-pocket costs for individuals with atopic eczema: a cross-sectional study in nine European countries. Acta Derm Venereol 2019; 99: 263–267.
- Mokkink LB, de Vet HCW, Prinsen CAC, Patrick DL, Alonso J, Bouter LM, et al. COSMIN risk of bias checklist for systematic reviews of patient-reported outcome measures. Qual Life Res 2018; 27: 1171–1179.
- Stuart BL, Howells L, Pattinson RL, Chalmers JR, Grindlay D, Rogers NK, et al. Measurement properties of patient-reported outcome measures for eczema control: a systematic review. J Eur Acad Dermatol Venereol 2021; 35: 1987–1993.