ORIGINAL REPORT
A Randomized Clinical Trial to Evaluate the Efficacy of an Oral Probiotic in Acne Vulgaris
Cristina EGUREN1, Ariadna NAVARRO-BLASCO2, Marina CORRAL-FORTEZA3, Alejandra REOLID-PÉREZ1, Núria SETÓ-TORRENT3, Alejandro GARCÍA-NAVARRO2, David PRIETO-MERINO4, Eva NÚÑEZ-DELEGIDO5, Pedro SÁNCHEZ-PELLICER5 and Vicente NAVARRO-LÓPEZ5,6
1Department of Dermatology, Eguren Dermatology and Aesthetics Clinic, Madrid, Spain, 2Department of Dermatology, Dermatological and Aesthetic Center, Alicante, Spain, 3Department of Dermatology, University Hospital Sagrat Cor, Barcelona, Spain, 4Faculty of Medicine, University of Alcalá de Henares, Madrid, Spain, 5Faculty of Medicine, Catholic University of Murcia (UCAM), Murcia, Spain, 6Department of Internal Medicine, University Hospital Vinalopó-Fisabio, Elche, Spain
The relevance of the gut microbiota in some skin inflammatory diseases, including acne vulgaris, has been emphasized. Probiotics could play a role in the modulation of the microbiota, improving the clinical course of this disease. A 12-week randomized, double-blind, placebo-controlled, clinical trial with patients aged 12 to 30 years with acne vulgaris was conducted. The study product was a capsule composed of the probiotic Lacticaseibacillus rhamnosus (CECT 30031) and the cyanobacterium Arthrospira platensis (BEA_IDA_0074B). Patients with improvement in the Acne Global Severity Scale were 10/34 (29.41%) in the placebo group compared with 20/40 (50%) in the probiotic group (p = 0.03). A significant reduction (p = 0.03) in the number of non-inflammatory acne lesions was observed in the probiotic group (–18.60 [–24.38 to –12.82]) vs the placebo group (–10.54 [–17.43 to –3.66]). Regarding the number of total lesions, a reduction almost reaching statistical significance (p = 0.06) was observed in the probiotic group (–27.94 [–36.35 to –19.53]) compared with the placebo group (–18.31 [–28.21 to –8.41]). In addition, patients with improvement attending the Global Acne Grading System were 7/34 (20.58%) in the placebo group vs 17/40 (42.50%) in the probiotic group (p = 0.02). The number of adverse events was similar in both groups. The probiotic used in this study was effective and well tolerated, and it should be considered for acne vulgaris patients.
Key words: acne vulgaris; dermatology; clinical trial; clinical study; microbiota; probiotics.
SIGNIFICANCE
Acne vulgaris is a common skin disease that significantly impairs the patient’s quality of life and affects their mental health. The gut–skin axis implies a bidirectional communication between skin and intestinal functions. Probiotics, “live microorganisms which when administered in adequate amounts confer a health benefit on the host”, have effects in the gut microbial function benefiting this key communication. The results of this clinical trial suggest that the probiotic mixture included in this study improves the clinical course in patients with acne vulgaris, supporting the use of this type of treatment and expanding the therapeutic arsenal.
Citation: Acta Derm Venereol 2024; 104: adv33206. DOI https://doi.org/10.2340/actadv.v104.33206.
Copyright: © 2024 The Author(s). Published by MJS Publishing, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/).
Submitted: Dec 1, 2023; Accepted: Apr 11, 2024; Published: May 15, 2024
Corr: Vicente Navarro-López, Faculty of Medicine, Catholic University of Murcia (UCAM), Avenida de los Jerónimos 135, Guadalupe, CP 30107 Murcia, Spain. E-mail: vnavarro@ucam.edu
Competing interests and funding: EN-D, PS-P and VN-L are employees of Bioithas (a sister company of Bionou Research) and VN-L owns stock/stock options.
INTRODUCTION
Acne vulgaris (AV) is one of the most common skin pathologies, with an estimated global prevalence at any age of 8.96% in men and 9.81% in women, according to the Global Burden of Disease Study 2010 (1). AV begins and progresses during adolescence, where the peak prevalence is reached, with variation in rates depending on the country and epidemiological study designs (2). Nevertheless, about 40% of adults who experience AV during adolescence may still present it in their 30s and 40s (3).
AV is an inflammatory skin disease affecting the pilosebaceous unit. Immunological, genetic, and hormonal factors are involved in its pathogenesis (4). Hyperproliferation and abnormal differentiation of keratinocytes (hyperkeratinization) (4), excess of sebum production (5), androgenic receptor activation in sebocytes and follicular keratinocytes (6, 7), infiltration of inflammatory cells (7), and skin colonization by some specific strains with pathogenic properties of Cutibacterium acnes (8–13) are key processes in the triggering and progression of AV.
Mild to moderate forms of AV are usually treated with topical drugs with antibiotic and anti-inflammatory activity such as topical retinoids, hormonal antiandrogens, benzoyl peroxide, and topical clindamycin, among others. For more severe cases, retinoids and oral antibiotics are indicated (14). Long-term administration of these medications implies the potential risk of increasing microbial resistance to antibiotics becoming ineffective (15), and safety risks associated with their potential adverse effects (16). Thus, development of safer alternative treatments with more durable efficacy should be a priority as it could significantly improve the quality of life of millions of people around the world.
In recent years, the importance of the gut microbiota in the shaping of the immune response and its key role in the gut–skin axis has been emphasized (17), because of the development of next generation sequencing (NGS) technology, including 16S rRNA gene sequencing (18). This has enabled the taxonomical characterization of a greater range of bacteria than microbiological cultures (19). Several studies have reported that AV patients present gut dysbiosis with reductions in diversity, richness, and short chain fatty acids (SCFAs)-producing bacteria (20, 21). However, mechanisms by which the gut microbiota influence AV progression have not been fully elucidated. In this regard, SCFAs are an influential class of bacterial metabolites derived from the anaerobic fermentation of dietary complex polysaccharides, which can directly activate G-coupled receptors, inhibit histone deacetylases, serve as energy substrates, and thus affect various physiological processes (22). Many studies have supported the influence of SCFAs on the balance between Treg and Th17 lymphocytes (23). Activation of Th17 cells by different mechanisms is a key process in AV pathogenesis, by establishing an inflammatory process in acne lesions with release of cytokines that cause neutrophilic infiltration in the pilosebaceous follicle (7). In addition, SCFAs protect against increased intestinal permeability (24), which is associated with bacterial translocation, low-grade inflammation, accumulation of metabolites in the skin, and disruption of skin homeostasis, which could disrupt the cutaneous microbiota (17).
The modulation of the gut microbiota through the administration of probiotics, as adjuvant or alternative therapy, could improve the clinical course of AV and reduce the adverse effects of a conventional therapy. This approach has achieved positive results with other inflammatory skin diseases such as atopic dermatitis or psoriasis (25). Nevertheless, clinical trials with probiotic, symbiotic, or postbiotic interventions in AV patients are scarce (4), although the results are generally encouraging (26–30). The aim of the present study was to determine the efficacy and safety of a probiotic preparation on the clinical course of AV patients when administered as adjuvant treatment.
MATERIALS AND METHODS
Study design
This clinical trial had a randomized, double-blind, placebo-controlled design with a 12-week treatment. The study protocol was evaluated and approved by the ethics committees from the University Hospital Sagrat Cor (Barcelona, Spain) and UCAM (Murcia, Spain) and registered in the American Registry of Clinical Trials (Clinicaltrials.gov identifier: NCT04570319). The study was structured in 4 face-to-face visits when the different procedures for outcomes measures were performed; a baseline visit and at 4, 8, and 12 weeks after starting treatment.
Participant selection criteria
Subjects with AV between 12 and 30 years old with a minimum severity category of “mild” according to Acne Global Severity Scale (AGSS) were included. An obligatory requirement was the signature of the informed consent form (in the case of minors, the signature was also provided by a legal guardian). In addition, the patients received a list of dietary recommendations that they had to follow during the study. Subjects with a contraindication to any of the components of the study product, and those who had consumed probiotics in the previous 2 months or had been treated with systemic retinoids in the previous 6 months, were excluded.
Recruitment and randomization
AV patients evaluated from October 2020 to April 2022 in several Spanish dermatological clinics were offered participation in the study. Those who accepted, signed the informed consent, and met the total inclusion criteria and none of the exclusion criteria were randomly assigned to each of the 2 intervention groups in a 1:1 ratio, following a randomized list previously prepared by blinded personnel.
Treatments
The study product is a lyophilized preparation composed of the probiotic Lacticaseibacillus rhamnosus (formerly Lactobacillus rhamnosus) (CECT 30031) and the cyanobacterium Arthrospira platensis (BEA_IDA_0074B) with a concentration of 1x109 colony-forming units (cfu) per daily dose. This blend is formulated with maltodextrin as a carrier in a hydroxypropyl methylcellulose capsule, being prescribed once daily. The safety of both bacteria was considered to be guaranteed due to their inclusion in the Qualified Presumption of Safety (QPS) list of the European Food Safety Authority (EFSA). A placebo with the same appearance as the active product, containing only maltodextrin, was used as comparator product.
Briefly, the selection of this specific strain of Lacticaseibacillus rhamnosus was made based on unpublished in vitro studies together with reported beneficial effects of other Lacticaseibacillus rhamnosus strains improving intestinal permeability (31), gut dysbiosis (32), appropriate balance between Th17 and Treg lymphocytes (33), and decreased proinflammatory cytokines (34). Arthrospira platensis BEA_IDA_0074B was selected because of its unique ability to produce large amounts of cyano-phycocyanin compared with other strains (unpublished data). Some reported skin beneficial properties of cyano-phycocyanin are antimicrobial activity against Cutibacterium acnes (35) and anti-inflammatory effects (36).
The investigators were instructed to follow consistently the recommendations of the clinical guideline for management of mild and moderate AV cases of the European Academy of Dermatology and Venerology (14). All researchers were requested that treatments remain unchanged during the 12-week intervention period.
Outcomes and procedures
The primary outcome was the difference between the 2 study groups in the number and percentage of patients who improve regarding AGSS category. This improvement was defined as the change in at least 1 category of less severity (e.g. upgrade from “moderate” to “mild”, “mild” to “almost clean”, or from “almost clean” to “clean”) when comparing values between baseline and after 12 weeks’ follow-up. As a secondary outcome the difference between study groups in the number and percentage of patients with clinically significant improvement of at least 30% in Global Acne Grading System (GAGS) score was established, when comparing values between baseline and after 12 weeks’ follow-up. Another secondary outcome was also the change between probiotic and placebo groups in the count of total, non-inflammatory, and inflammatory AV lesions, between baseline and after 12 weeks’ follow-up. In addition, the count, type, severity, and causality in relation to the study product of all adverse effects (AEs) that resulted in the 12-weeks intervention period were collected.
Statistical analysis
A sample size of 40 patients per group was estimated to recognize as statistically significant a difference greater than 27% of patients with clinical improvement of at least 1 category of less severity of AGSS (primary outcome), accepting an alpha risk of 0.05 and a beta risk of 0.2 in a one-sided test. Moreover, a dropout rate of 10% was anticipated.
Data were analysed as intention-to-treat. Descriptive statistics for quantitative variables were presented as mean and 95% confidence interval, and categorical variables as total and proportion of cases. Logistic regression was used for analysis of categorical variables. These regression models were adjusted by the baseline category (i.e. “AGSS category” at baseline) and sex of the patients, because some studies have reported that women with AV are more sensitive than men regarding their skin condition (37). In addition, a linear mixed-effects model was used for analysis of continuous variables. Each of these models was adjusted by the baseline value of the variable (i.e. “number of total lesions” at baseline), sex, and AGSS baseline category of the patients. A random effect term by patient was included by account for repeated measures. Statistical significance was defined as p < 0.05.
RESULTS
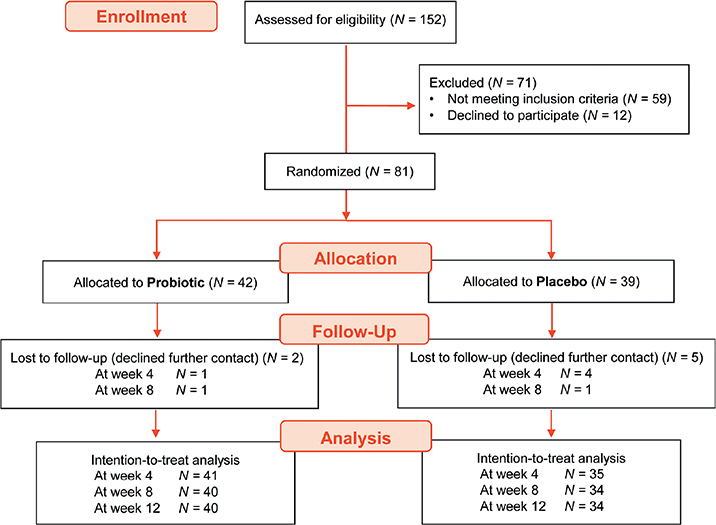
The information on the subjects evaluated for eligibility, those randomized, and finally the cases analyzed is included in the CONSORT diagram (Fig. 1). The baseline characteristics of the patients included in the study in each intervention group are described in Table I.
Acne Global Severity Scale (AGSS)
A higher number of patients with improvement of at least 1 category of less severity at the end of the study regarding AGSS occured in the probiotic group compared with the placebo group: 20 of 40 (50.00 %) vs 10 of 34 (29.41 %). The applied logistic regression model established this difference between groups at the end of the intervention period as statistically significant (p = 0.03). Fig. 2 illustrates the progression of the response according to AGSS at 4, 8, and 12 weeks of intervention in the probiotic and placebo groups. Interestingly, a post-hoc analysis of these data revealed that 9 of 40 (22.50%) subjects in the probiotic group finished (after 12 weeks) in the less severe categories (“clean” and “almost clean”), while this occurred in only 2 of 34 (5.88%) patients in the placebo group. Separate analysis of the patients classified at baseline in the “mild” category shows a higher response rate in the probiotic when compared with the placebo group (20% vs 5%), while in patients classified in the “moderate/severe” categories, the response rate was also higher in the probiotic group (70% compared with 43%).
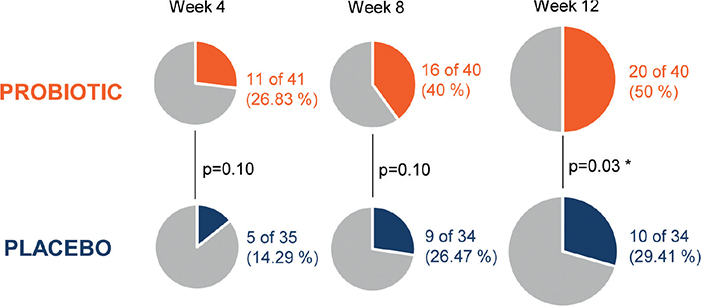
Fig. 2. Evolution of the Acne Global Severity Scale (AGSS). Patients with improvement in at least 1 category of less severity during the study in both intervention groups. Since as early as week 4 of treatment the proportion of patients with clinical response according to AGSS was higher in the probiotic group compared with the placebo group. This difference was statistically significant when treatment was completed after 12 weeks of intervention.
Total, non-inflammatory, and inflammatory acne vulgaris lesions
A decrease in the number of AV lesions, whether total, inflammatory, or non-inflammatory, was always observed in the probiotic group compared with the placebo group after 12 weeks of intervention. In the case of non-inflammatory AV lesions this difference (–8.06) was statistically significant at the end of the study (p = 0.03). However, although inflammatory AV lesions count presents a higher reduction in the probiotic group (–2.54), this difference compared with the placebo group was not statistically significant at the end of the study. Finally, regarding total AV lesions count, a clinically relevant reduction is shown in the probiotic group compared with the placebo group (–9.63), which almost reaches statistical significance at the end of the study (p = 0.06). Table II summarizes data analysis of the total, non-inflammatory, and inflammatory AV lesions.
Global Acne Grading System (GAGS)
A superior clinical response based on GAGS was observed in patients enrolled in the probiotic group, similar to what was reported with AGSS. Therefore, a higher number of patients with improvement of at least 30% in GAGS score at the end of the study occurred in the probiotic group compared with the placebo group: 17 of 40 (42.50%) vs 7 of 34 (20.58%). The applied logistic regression model established this difference between groups at the end of the intervention period as statistically significant (p = 0.02). Table III summarizes data of patients with clinical response based on GAGS score.
Safety
All AEs occurred during the study were registered and classified according to the affected system (Table IV). There were no differences in the number of AEs per study group in any follow-up period (weeks 4, 8, and 12). All reported cases of AEs were categorized as mild severity. Seven AEs were attributed only to the study product, being 3 in the probiotic group and 4 in the placebo group; all affected the digestive system.
DISCUSSION
Some in vitro studies have shown several interesting properties of probiotic strains regarding their ability to produce antimicrobial substances that inhibit the growth of Cutibacterium acnes, suggesting its potential effect in the treatment of AV patients (4). However, to date, human clinical trials that used oral probiotics have been scarce, and there is no strong experimental evidence supporting strain-specific effectiveness and safety in clinical practice.
Overall, the present clinical trial suggests that adjuvant probiotic treatment improves the clinical management of patients with mild and moderate forms of AV in a general clinical practice setting. A higher percentage of patients receiving the probiotic treatment improved in at least 1 AGSS category of less severity, with a statistically significant difference when comparing with the placebo group after 12 weeks of intervention. A similar result was obtained in favour of the probiotic preparation when the clinical response is analysed according to GAGS score outcome. Likewise, the number of total lesions presents a higher reduction in patients treated with the probiotic mixture, where differences reach quasi-statistical significance, mainly through the significant reduction of non-inflammatory lesions. Regarding inflammatory lesions, although the reduction is more pronounced in the probiotic group and the difference between both treatment groups increases at each monitoring point during the study, there is no statistically significant difference between groups at the end of the study. In this last variable, the increase in difference between groups is greater the longer the intervention time, suggesting that probiotic treatment for longer than 12 weeks would be necessary to observe a statistically significant difference in acne inflammatory lesions, and there might have been a greater difference between the groups in the other efficacy variables if the treatment had been maintained for longer than 12 weeks.
Other clinical trials using probiotics in cases of AV, although of different design or procedures, have reported positive results in the clinical course of AV patients. Jung et al. reported a preliminary study of 45 mild to moderate AV patients treated with a probiotic mixture, a probiotic mixture plus minocycline, or with minocycline, all of them in combination with standard topical medication. After 12 weeks of treatment, the highest reduction in the number of acne lesions count was achieved in patients treated with the probiotic plus minocycline, but statistical analysis and how the results are evaluated are not clear in the article (26). In another study, 20 patients with active inflammatory AV on the back were randomized to receive only treatment with the probiotic strain Lactobacillus rhamnosus SP1 or placebo. Patients in the probiotic group were much more likely to be classified by a specialist physician with the rating “improved” or “markedly improved” in respect of their AV lesions (27).
The intervention probiotic selected in our study is composed of Lacticaseibacillus rhamnosus CECT 30031 strain and cyanobacterium Arthrospira platensis BEA_IDA_0074B strain. Fabbrocini et al. (27), using a different strain of Lactobacillus rhamnosus, observed a reduction in IGF-1 gene expression and an increase in FoxO1 gene expression in skin AV areas. These findings could imply reduced activation of androgen receptors in the skin, which is an important pathogenic mechanism in AV (38, 39).
Some limitations of the study should be considered. Diet was not analysed as a variable, although it is an acknowledged factor that participates in the pathogenesis of AV (7, 40). However, patients were provided with a list of hyperglycaemic, dairy, and fatty foods to be avoided. Another limitation is the applicability of the results to the probiotic product used in this study to a young adolescent population under 12 years and an adult population older than 30 years not included in the present study. In this respect, persistent or late-onset AV is more frequent in women than in men (41). Presumably, in these women the most influential pathogenic mechanism is increased production of androgens in the skin or even increased receptor androgen sensitivity (4). Adjuvant treatment with probiotics in these cases has not been studied. Therefore, it is not demonstrable that the probiotic is effective above this age range.
In conclusion, the probiotic formula investigated as an adjuvant treatment in a routine clinical practice scenario improves the clinical course of patients with AV. The probiotic treatment was safe and effective with clinical and statistically significant reduction in the severity of AV.
ACKNOWLEDGEMENTS
This study was supported by Bionou Research. The role of the funding organization was design of the study, approval of the manuscript, and decision to submit the manuscript for publication.
IRB approval status
The study protocol was approved at each study site. A total of 4 review boards or ethics committees reviewed and approved the study protocols.
REFERENCES
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2163–2196.
- Heng AHS, Chew FT. Systematic review of the epidemiology of acne vulgaris. Sci Rep 2020; 10: 5754.
- Bhate K, Williams HC. Epidemiology of acne vulgaris. Br J Dermatol 2013; 168: 474–485.
- Sánchez-Pellicer P, Navarro-Moratalla L, Núñez-Delegido E, Ruzafa-Costas B, Agüera-Santos J, Navarro-López V. Acne, microbiome, and probiotics: the gut–skin axis. Microorganisms 2022; 10: 1303.
- Ottaviani M, Camera E, Picardo M. Lipid mediators in acne. Mediators Inflamm 2010; 2010: 858176.
- Hu T, Wei Z, Ju Q, Chen W. Sex hormones and acne: state of the art. J Dtsch Dermatol Ges 2021; 19: 509–515.
- Melnik BC. Linking diet to acne metabolomics, inflammation, and comedogenesis: an update. Clin Cosmet Investig Dermatol 2015; 8: 371–388.
- Kwon HH, Yoon JY, Park SY, Suh DH. Analysis of distribution patterns of Propionibacterium acnes phylotypes and Peptostreptococcus species from acne lesions. Br J Dermatol 2013; 169: 1152–1155.
- Barnard E, Shi B, Kang D, Craft N, Li H. The balance of metagenomic elements shapes the skin microbiome in acne and health. Sci Rep 2016; 6: 39491.
- Johnson T, Kang D, Barnard E, Li H. Strain-level differences in porphyrin production and regulation in propionibacterium acnes elucidate disease associations. mSphere 2016; 1: e00023-15.
- Coenye T, Spittaels KJ, Achermann Y. The role of biofilm formation in the pathogenesis and antimicrobial susceptibility of cutibacterium acnes. Biofilm 2022; 4: 100063.
- Jahns AC, Lundskog B, Ganceviciene R, Palmer RH, Golovleva I, Zouboulis CC, et al. An increased incidence of Propionibacterium acnes biofilms in acne vulgaris: a case-control study. Br J Dermatol 2012; 167: 50–58.
- Yu Y, Champer J, Agak GW, Kao S, Modlin RL, Kim J. Different Propionibacterium acnes phylotypes induce distinct immune responses and express unique surface and secreted proteomes. J Invest Dermatol 2016;1 36: 2221–2228.
- Nast A, Dréno B, Bettoli V, Bukvic Mokos Z, Degitz K, Dressler C, et al. European evidence-based (S3) guideline for the treatment of acne – update 2016 – short version. J Eur Acad Dermatol Venereol 2016; 30: 1261–1268.
- Walsh TR, Efthimiou J, Dréno B. Systematic review of antibiotic resistance in acne: an increasing topical and oral threat. Lancet Infect Dis 2016; 16: e23–33.
- Garner SE, Eady A, Bennett C, Newton JN, Thomas K, Popescu CM. Minocycline for acne vulgaris: efficacy and safety. Cochrane Database Syst Rev 2012; 2012: CD002086.
- Salem I, Ramser A, Isham N, Ghannoum MA. The gut microbiome as a major regulator of the gut–skin axis. Front Microbiol 2018; 9: 1459.
- Gilbert JA, Blaser MJ, Caporaso JG, Jansson JK, Lynch SV, Knight R. Current understanding of the human microbiome. Nat Med 2018; 24: 392–400.
- Wensel CR, Pluznick JL, Salzberg SL, Sears CL. Next-generation sequencing: insights to advance clinical investigations of the microbiome. J Clin Invest 2022; 132: e154944.
- Deng Y, Wang H, Zhou J, Mou Y, Wang G, Xiong X. Patients with acne vulgaris have a distinct gut microbiota in comparison with healthy controls. Acta Derm Venereol 2018; 98: 783–790.
- Huang Y, Liu L, Chen L, Zhou L, Xiong X, Deng Y. Gender-specific differences in gut microbiota composition associated with microbial metabolites for patients with acne vulgaris. Ann Dermatol 2021; 33: 531–540.
- Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 2016; 165: 1332–1345.
- Kim CH. Control of lymphocyte functions by gut microbiota-derived short-chain fatty acids. Cell Mol Immunol 2021; 18: 1161–1171.
- Usuda H, Okamoto T, Wada K. leaky gut: effect of dietary fiber and fats on microbiome and intestinal barrier. Int J Mol Sci 2021; 22: 7613.
- Navarro-López V, Núñez-Delegido E, Ruzafa-Costas B, Sánchez-Pellicer P, Agüera-Santos J, Navarro-Moratalla L. Probiotics in the therapeutic arsenal of dermatologists. Microorganisms 2021; 9: 1513.
- Jung GW, Tse JE, Guiha I, Rao J. Prospective, randomized, open-label trial comparing the safety, efficacy, and tolerability of an acne treatment regimen with and without a probiotic supplement and minocycline in subjects with mild to moderate acne. J Cutan Med Surg 2013; 17: 114–122.
- Fabbrocini G, Bertona M, Picazo Ó, Pareja-Galeano H, Monfrecola G, Emanuele E. Supplementation with Lactobacillus rhamnosus SP1 normalises skin expression of genes implicated in insulin signalling and improves adult acne. Benef Microbes 2016; 7: 625–630.
- Tolino E, Skroza N, Mambrin A, Bernardini N, Zuber S, Balduzzi V, et al. Novel combination for the treatment of acne differentiated based on gender: a new step towards personalized treatment. G Ital Dermatol Venereol 2018; 153: 866–871.
- Manzhalii E, Hornuss D, Stremmel W. Intestinal-borne dermatoses significantly improved by oral application of Escherichia coli Nissle 1917. World J Gastroenterol 2016; 22: 5415–5421.
- Rinaldi F, Marotta L, Mascolo A, Amoruso A, Pane M, Giuliani G, et al. Facial acne: a randomized, double-blind, placebo-controlled study on the clinical efficacy of a symbiotic dietary supplement. Dermatol Ther (Heidelb) 2022; 12: 577–589.
- Chen L, Li H, Li J, Chen Y, Yang Y. Lactobacillus rhamnosus GG treatment improves intestinal permeability and modulates microbiota dysbiosis in an experimental model of sepsis. Int J Mol Med 2019; 43: 1139–1148.
- Panpetch W, Hiengrach P, Nilgate S, Tumwasorn S, Somboonna N, Wilantho A, et al. Additional candida albicans administration enhances the severity of dextran sulfate solution induced colitis mouse model through leaky gut-enhanced systemic inflammation and gut-dysbiosis but attenuated by Lactobacillus rhamnosus L34. Gut Microbes 2020; 11: 465–480.
- Jia L, Wu R, Han N, Fu J, Luo Z, Guo L, et al. Porphyromonas gingivalis and Lactobacillus rhamnosus GG regulate the Th17/Treg balance in colitis via TLR4 and TLR2. Clin Transl Immunology 2020; 9: e1213.
- Chen JF, Zhuang Y, Jin SB, Zhang SL, Yang WW. Probiotic lactobacillus rhamnosus GG (LGG) restores intestinal dysbacteriosis to alleviate upregulated inflammatory cytokines triggered by femoral diaphyseal fracture in adolescent rodent model. Eur Rev Med Pharmacol Sci 2021; 25: 376–389.
- Nihal B, Gupta NV, Gowda D, Mariapan M. Formulation and development of topical anti acne formulation of spirulina extract. Int J Appl Pharm 2018; 10: 229–233.
- Dranseikienė D, Balčiūnaitė-Murzienė G, Karosienė J, Morudov D, Juodžiukynienė N, Hudz N, et al. Cyano- phycocyanIn: mechanisms of action on human skin and future perspectives in medicine. Plants (Basel) 2022; 11: 1249.
- Kellett SC, Gawkrodger DJ. The psychological and emotional impact of acne and the effect of treatment with isotretinoin. Br J Dermatol 1999; 140: 273–282.
- Mirdamadi Y, Bommhardt U, Goihl A, Guttek K, Zouboulis CC, Quist S, et al. Insulin and insulin-like growth factor-1 can activate the phosphoinositide-3-kinase /Akt/FoxO1 pathway in T cells in vitro. Dermatoendocrinol 2017; 9: e1356518.
- Melnik BC. The role of transcription factor FoxO1 in the pathogenesis of acne vulgaris and the mode of isotretinoin action. G Ital Dermatol Venereol 2010; 145: 559–571.
- Dall’Oglio F, Nasca MR, Fiorentini F, Micali G. Diet and acne: review of the evidence from 2009 to 2020. Int J Dermatol 2021; 60: 672–685.
- Rocha MA, Bagatin E. Adult-onset acne: prevalence, impact, and management challenges. Clin Cosmet Investig Dermatol 2018; 11: 59–69.