SHORT COMMUNICATION
Ustekinumab Demonstrates Lower Uveitis Risk in Moderate to Severe Psoriasis Patients Compared with Tumor Necrosis Factor-α Inhibitors
Chul Hwan BANG1, Hyun Ju OH1, Yeong Ho KIM1, Jin-Hyung JUNG2, Ji Hyun LEE1, Young Min PARK1 and Ju Hee HAN1*
1Department of Dermatology, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, 222 Banpodaero, Seocho-gu, Seoul, 06591, Republic of Korea, and 2Department of Biostatistics, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea. *E-mail: alwaysmine8@gmail.com
Citation: Acta Derm Venereol 2024; 104: adv34206. DOI: https://doi.org/10.2340/actadv.v104.34206.
Copyright: © 2024 The Author(s). Published by MJS Publishing, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/).
Submitted: Dec 4, 2023; Accepted after revision: Jul 3, 2024; Published: Sep 9, 2024
Competing interests and funding: The authors have no conflicts of interest to declare.
INTRODUCTION
Uveitis is inflammation of the uvea and is closely associated with immune-mediated inflammatory disease, which can cause permanent vision loss. Recent studies have reported an increase in the incidence of uveitis among psoriasis patients, and the Joint AAD-NPF guidelines recommend that dermatologists actively participate in early detection of uveitis (1, 2). A recent study by Kim et al. (3) reported that the rates of uveitis incidence and uveitis recurrence in patients with psoriasis were 1.18 and 2.31 per 1,000 person-years, respectively. However, the study on the incidence rate of uveitis among individuals treated with each medication was limited (4). In this study, we compared the risk of uveitis in patients being treated with tumour necrosis factor-α inhibitors (TNFi) compared with that in those receiving ustekinumab.
METHODS
We included patients with moderate to severe psoriasis who had a psoriasis area and severity index score > 10, despite receiving conventional treatment for 3 months between January 2016 and September 2019 from the Korean Health Insurance Review and Assessment Service database. The study was reviewed and approved by the Institutional Review Board of the Catholic University of Korea (approval: KC20ZISI0189). Specifically, we identified patients with psoriasis who were using ustekinumab or TNFi. After excluding patients who had a prior diagnosis of uveitis (H20, H22), we included 2,467 patients receiving ustekinumab and 1,746 patients receiving TNFi. The risk of uveitis in groups treated with ustekinumab or TNFi was analysed using a Cox regression hazard model. We adjusted for age, sex, diabetes, hypertension, dyslipidaemia, malignancy, and psoriatic arthritis in the regression models. Additionally, we evaluated the hazard ratio by adjusting for covariates using inverse probability of treatment weighting (IPTW) (Table I).
| Number | Event | Person-years | IR* | HR (95% CI) | |||||
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |||||
| Before IPTW | |||||||||
| TNF-α inhibitor | 1,746 | 35 | 3,446.6 | 10.16 | 1 [Reference]** | 1 | 1 | 1 | 1 |
| Ustekinumab | 2,467 | 13 | 5,551.5 | 2.34 | 0.232 (0.123–0.439) | 0.226 (0.119–0.428) | 0.223 (0.118–0.424) | 0.222 (0.117–0.421) | 0.223 (0.117–0.424) |
| After IPTW | |||||||||
| TNF-α inhibitor | 1,784.6 | 35.4 | 3,481.4 | 10.18 | 1 | ||||
| Ustekinumab | 2,464.6 | 12.6 | 5,515.7 | 2.28 | 0.223 (0.113–0.414) | ||||
| *Per 1,000 person-years. **TNFi is the reference. | |||||||||
| Model 1: non-adjusted. Model 2: Adjusted for sex and age. Model 3: Adjusted for sex, age, diabetes mellitus, hypertension, and dyslipidaemia. Model 4: Adjusted for sex, age, diabetes mellitus, hypertension, dyslipidaemia, and malignancy. Model 5: Adjusted for sex, age, diabetes mellitus, hypertension, dyslipidaemia, malignancy, and psoriatic arthritis. | |||||||||
| IR: incidence rate; HR: hazard ratio; CI: confidence interval. | |||||||||
RESULTS
The incidence rates of uveitis were 10.16 and 2.34 per 1,000 person-years in the TNFi and ustekinumab groups, respectively (see Table I). The ustekinumab group had a significantly lower risk of uveitis compared with the TNFi group, with a hazard ratio (HR) of 0.22 (95% confidence interval [CI]: 0.113–0.414). Furthermore, multivariable Cox proportional hazards regression analysis confirmed a lower risk of uveitis in the ustekinumab group (HR, 0.22; 95% CI, 0.117–0.424) in comparison with the TNFi group. Also, the ustekinumab group had a significantly lower cumulative incidence rate of uveitis than the TNFi group (log-rank test, p < 0.001) (Fig. 1).
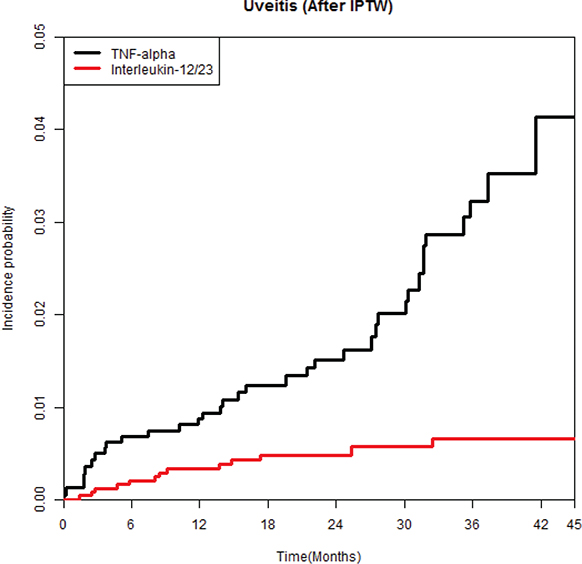
Fig. 1. Weighted cumulative incidence curves of uveitis in patients treated with ustekinumab or tumour necrosis factor-α inhibitors.
DISCUSSION
In our study, ustekinumab demonstrated a lower risk of uveitis in patients with moderate to severe psoriasis compared with TNFi. The observed lower incidence of uveitis in patients receiving ustekinumab may be attributed to its distinct mechanism of action. It is known that Th-1/Th-17 cells play roles in the development of both psoriasis and uveitis and IL-23 is one of the essential cytokines related to this inflammatory pathway. As inhibition of IL-23 prevents the maintenance of IL-17 and the inflammatory pathway of psoriasis, ustekinumab may be more effective than TNFi in preventing uveitis. For similar reasons, ustekinumab appears to have better preventative effects than TNFi in psoriatic arthritis and heart failure (5, 6). Moreover, some case series have reported the development of paradoxical uveitis after using TNFi in the treatment of psoriasis (7). Therefore, in patients with moderate to severe psoriasis who have risk factors for uveitis, ustekinumab might be a better option than TNFi.
The limitation of this study is that the diagnoses of psoriasis and uveitis were assessed using the ICD-10 codes in a health-insurance claims database. The reliance on ICD-10 codes from a health insurance claims database for diagnosing psoriasis and uveitis can introduce misclassification errors, as coding may not always be accurate or up to date. Moreover, an inability to extract and analyse information on the main features of the study populations from the database is also a limitation. Nevertheless, the strength of our study is that we conducted a nationwide cohort study on uveitis in psoriasis patients. Also, to the best of our knowledge, while there have been many studies on the association between psoriasis and uveitis, this is the first study to investigate the incidence rates of uveitis for different psoriasis treatments.
Of the many biologics developed for psoriasis, only a few have proved to be effective for various comorbidities. Therefore, our findings provide valuable insights into the potential benefits of ustekinumab as a therapeutic option for psoriasis patients as compared with TNFi. Further research is needed to investigate the mechanisms by which ustekinumab demonstrates lower risk of uveitis and to determine whether other biological agents that inhibit the IL-23/17 axis have a preventive effect on uveitis.
ACKNOWLEDGEMENTS
Data sharing statement: Data available on request from the authors.
REFERENCES
- Egeberg A, Khalid U, Gislason GH, Mallbris L, Skov L, Hansen PR. Association of psoriatic disease with uveitis: a Danish nationwide cohort study. JAMA Dermatol 2015; 151: 1200–1205. https://doi.org/10.1001/jamadermatol.2015.1986
- Elmets CA, Leonardi CL, Davis DMR, Gelfand JM, Lichten J, Mehta NN, et al. Joint AAD–NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol 2019; 80: 1073–1113. https://doi.org/10.1016/j.jaad.2018.11.058
- Kim BR, Choi SW, Choi CW, Lee KH, Kim MJ, Woo SJ, et al. Risk of uveitis in patients with psoriasis in Korea: a nationwide population-based cohort study. J Eur Acad Dermatol Venereol 2023; 37: 1336–1343. https://doi.org/10.1111/jdv.19060
- Jadon DR, Corp N, van der Windt DA, Coates LC, Soriano ER, Kavanaugh A, et al. Management of concomitant inflammatory bowel disease or uveitis in patients with psoriatic arthritis: an updated review informing the 2021 GRAPPA Treatment Recommendations. J Rheumatol 2023; 50: 438–450. https://doi.org/10.3899/jrheum.220317
- Singla S, Putman M, Liew J, Gordon K. Association between biological immunotherapy for psoriasis and time to incident inflammatory arthritis: a retrospective cohort study. Lancet Rheumatol 2023; 5: e200–e207. https://doi.org/10.1016/S2665-9913(23)00034-6
- Han JH, Park HE, Kim YH, Jung JH, Lee JH, Park YM, et al. Comparison of the risk of heart failure in psoriasis patients using anti-TNF α inhibitors and ustekinumab. ESC Heart Fail 2022; 9: 1502–1504. https://doi.org/10.1002/ehf2.13855
- Kose B, Uzlu D, Erdol H. Psoriasis and uveitis. Int Ophthalmol 2022; 42: 2303–2310. https://doi.org/10.1007/s10792-022-02225-5