ORIGINAL REPORT
Lebrikizumab Combined with Topical Corticosteroids Improves Patient-reported Outcomes in Japanese Patients with Moderate-to-severe Atopic Dermatitis
Akio TANAKA1, Ken IGAWA2, Hidetoshi TAKAHASHI3, Ryosuke SHIMIZU4, Yoko KATAOKA5, Hitoe TORISU-ITAKURA6, Yoji MORISAKI6, Sonia MONTMAYEUR7 and Norito KATOH8
1Department of Dermatology, Graduate School of Biomedical and Health Sciences, Hiroshima University, Hiroshima, 2Dokkyo Medical University, Dokkyo, 3Takagi Dermatology Clinic, 4Shimizu Dermatology Clinic, 5Osaka Habikino Medical Center, Osaka, 6Eli Lilly Japan K.K., Kobe, Japan, 7Eli Lilly and Company, Indianapolis, IN, USA, and 8Kyoto Prefectural University of Medicine, Kyoto, Japan
Lebrikizumab has previously demonstrated efficacy in Phase 3 trials: ADvocate1 and ADvocate2 (as monotherapy), ADhere, and ADhere-J (in combination with topical corticosteroids). Here, the impact of lebrikizumab combined with low- to mid-potency topical corticosteroids on patient-reported outcomes at 16 weeks in Japanese patients with moderate-to-severe atopic dermatitis is evaluated. Eligible patients (n = 286) were randomized 2:2:3 to receive placebo+ topical corticosteroids, 250 mg lebrikizumab every 4 weeks (LEBQ4W+topical corticosteroids, 500 mg loading dose at baseline), or 250 mg lebrikizumab every 2 weeks (LEBQ2W+ topical corticosteroids, 500 mg loading dose at baseline and Week 2) by subcutaneous injection. All PRO endpoints for the study were met; patients in the lebrikizumab in combination with topical corticosteroids groups demonstrated statistically significant and clinically meaningful improvements compared with placebo in combination with topical corticosteroids in Skin Pain NRS, DLQI, POEM, WPAI-AD, and SCORAD scales. Lebrikizumab combined with topical corticosteroids compared with placebo+topical corticosteroids improved patient-reported outcomes in Japanese patients with moderate-to-severe atopic dermatitis.
SIGNIFICANCE
Atopic dermatitis, a chronic skin disease, is one of the most common dermatological conditions in Japan. It has a high disease burden with intense itch and skin pain, impacting sleep and daily activities. Lebrikizumab is a monoclonal antibody that binds to interleukin-13, the key cytokine in atopic dermatitis, blocking its downstream effects. ADhere-J was a clinical trial of lebrikizumab in combination with topical corticosteroids in Japanese patients with moderate-to-severe atopic dermatitis. Here we report that lebrikizumab+topical corticosteroids significantly improved patient-reported outcomes related to skin pain, quality of life, sleep loss, and work productivity. These results support lebrikizumab as a promising treatment option in this population.
Key words: lebrikizumab; moderate-to-severe AD; Japanese patients; patient-reported outcomes.
Citation: Acta Derm Venereol 2024; 104: adv34375. DOI: https://doi.org/10.2340/actadv.v104.34375.
Copyright: © 2024 The Author(s). Published by MJS Publishing, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/).
Submitted: Dec 15, 2023; Accepted after revision: Jul 18, 2024; Published: Sep 9, 2024
Corr: Hitoe Torisu-Itakura, Bio-Medicines, Japan Drug Development and Medical Affairs, Eli Lilly Japan K.K., 5-1-28, Isogamidori, Chuo-ku Kobe-shi 651-0086 Japan. E-mail: itakura_hitoe@lilly.com
Competing interests and funding: AK has received lecturer honoraria from AbbVie GK, Eli Lilly Japan K.K., Kaken Pharmaceutical Co., Ltd., Kyorin Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Pfizer Japan Inc., Sanofi K.K., Taiho Pharmaceutical Co., Torii Pharmaceutical Co., Ltd., and Maruho Co., Ltd.; and has received research grants from Eli Lilly Japan K.K., Maruho Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Taiho Pharmaceutical Co., Teijin Pharma Limited, and Torii Pharmaceutical Co., Ltd.. KI has received lecture fees from AbbVie GK, Eli Lilly Japan K.K., Maruho Co., Ltd., Pfizer Japan Inc., and Sanofi K.K. HT has no conflicts of interest to declare. RS has received lecturer honoraria from AbbVie GK, Eli Lilly Japan K.K., LEO Pharma, Maruho Co., Ltd., Pfizer Japan Inc., Sanofi K.K., and Torii Pharmaceutical Co., Ltd. YK has received lecturer honoraria from AbbVie GK, Pfizer Japan Inc., and Sanofi K.K., and research funding from AbbVie GK, Amgen, Eli Lilly Japan K.K., LEO Pharma, Maruho Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Pfizer Japan Inc., Sanofi K.K., and Taiho Pharmaceutical Co., Ltd. HT-I and YM are employees of Eli Lilly Japan K.K. SM is an employee of Eli Lilly and Company. NK Katoh has received honoraria as a speaker/consultant for AbbVie GK, Eli Lilly Japan K.K., LEO Pharma, Maruho Co., Ltd., Pfizer Japan Inc., Sanofi K.K., and Taiho Pharmaceutical Co., Ltd., and has received grants as an investigator from AbbVie GK, LEO Pharma, Maruho Co., Ltd., Mitsubishi Tanabe Pharma Corporation, and Torii Pharmaceutical Co., Ltd.
This study was funded by Eli Lilly Japan K.K. Almirall, S.A. has licensed the rights to develop and commercialize lebrikizumab for the treatment of dermatology indications, including atopic dermatitis in Europe. Eli Lilly and Company has exclusive rights for development and commercialization of lebrikizumab in the United States and the rest of the world outside of Europe. Medical writing assistance was provided by Niamh Wiley, PhD, of Eli Lilly and Company.
INTRODUCTION
Atopic dermatitis (AD) is a chronic, relapsing, heterogeneous skin disease. It is one of the most common dermatological conditions in Japan (1, 2), with a prevalence of 6.9% in adults and 11.2% in elementary school children (3, 4). There is a high disease burden in Japanese patients as moderate-to-severe AD symptoms include intense itch and skin pain that affect sleep, daily activities, and social relationships (5, 6). The severity of disease symptoms correlates with poor quality of life and depression, and results in a negative impact on work productivity and daily activities (7, 8). Current therapeutic approaches for AD in Japan include topical corticosteroids (TCS), topical calcineurin inhibitors (TCI), topical Janus kinase (JAK) inhibitors, and topical phosphodiesterase-4 inhibitors for mild AD. For moderate-to-severe AD, dupilumab, tralokinumab, and lebrikizumab (recently approved), oral Janus kinase inhibitors, phototherapy, and systemic corticosteroids are prescribed, and cyclosporine is prescribed for very severe AD (9–11). However, additional systemic therapy options suitable for long-term management of moderate-to-severe AD are needed due to the heterogeneity of the disease (12).
Interleukin (IL)-13 is a pro-inflammatory cytokine that is central to AD pathogenesis and is overexpressed in patients with AD (13). It plays an important role in skin barrier dysfunction, skin infections, lichenification, itch, and inflammation (14). Patients with AD overexpress IL-13, and increased skin and serum IL-13 levels are associated with increased AD severity (15). Lebrikizumab is a novel, high-affinity monoclonal antibody that selectively binds to IL-13. Lebrikizumab prevents the formation of the IL-13Rα1/IL-4Rα heterodimer receptor signalling complex, thus blocking downstream IL-13 signalling. Lebrikizumab-bound IL-13 can still bind to the IL-13Rα2 receptor, allowing natural clearance of IL-13. Lebrikizumab exhibits high binding affinity, a slow dissociation rate, and neutralizes IL-13 with high potency (16). Lebrikizumab has previously demonstrated clinical efficacy in adult and adolescent patients with moderate-to-severe AD in 3 global Phase 3 trials: 2 52-week monotherapy studies (ADvocate1 [NCT04146363] and ADvocate2 [NCT04178967]) and a 16-week combination study with TCS (ADhere [NCT04250337]) (17, 18).
ADhere-J (NCT04760314) was a Phase 3 randomized, placebo-controlled 68-week clinical trial of lebrikizumab in combination with TCS in Japanese patients with moderate-to-severe AD. The aim of this analysis is to report the effect of lebrikizumab 250 mg every 4 weeks (Q4W) or lebrikizumab 250 mg every 2 weeks (Q2W) in combination with TCS compared with placebo in combination with TCS on patient-reported outcomes (PROs) at 16 weeks.
MATERIALS AND METHODS
Study design
ADhere-J was conducted at 37 centres from 10 March 2021 to 1 February 2023 and comprised a 68-week treatment period with induction (16 weeks) and 52-week maintenance periods, and a safety follow-up period of 10 weeks. The study protocol was approved by the local institutional review board or independent ethics committee at each study site and was conducted in accordance with the protocol, applicable ICH GCP guidelines, applicable laws and regulations, and consensus ethical principles derived from international guidelines, including the Declaration of Helsinki and Council for International Organizations of Medical Sciences International Ethical Guidelines. Written informed consent was obtained from each patient or legally authorized representative before enrolment.
Study population and eligibility criteria
Eligible patients were adults and adolescents (aged ≥ 12 to < 18 years, weighing ≥ 40 kg), had chronic AD according to the American Academy of Dermatology Consensus Criteria for ≥ 1 year before screening (19), had moderate-to-severe AD, defined as having an Eczema Area and Severity Index (EASI) score ≥ 16 (20), Investigator’s Global Assessment (IGA) score ≥ 3 (21), and body surface area (BSA) involvement ≥ 10% at baseline; and had a history of inadequate response to existing topical medications within 6 months before screening. Full eligibility criteria have been published previously1.
Treatment protocol
Patients were randomized 2:2:3 to receive placebo, lebrikizumab 250 mg Q4W (with 500 mg loading dose at baseline), or lebrikizumab 250 mg Q2W (with 500 mg loading dose at baseline and Week 2) in combination with TCS. All patients initiated mid-potency TCS (hydrocortisone butyrate 0.1% ointment or equivalent), and low-potency TCS (prednisolone 0.5% cream or equivalent) and/or TCI for sensitive areas only, ≥ 7 days before baseline, which could not be tapered or stopped until the baseline visit. After baseline, patients were permitted to stop or taper TCS based on treatment response. After the induction period, patients entered the 52-week maintenance period.
Use of medications for medical conditions that affect AD (including high-potency TCS, systemic corticosteroids, phototherapy, cyclosporine) and other systemic treatments were prohibited during the induction period and led to permanent discontinuation of study intervention. However, high-potency TCS used for intolerable AD symptoms or adverse events (AEs) were considered rescue treatments and patients continued the study intervention, attended all study visits to Week 16, and were eligible to enter the escape arm of the maintenance period. Patients who received systemic corticosteroids and other systemic treatments as rescue therapy discontinued the study intervention but could attend all study visits to Week 16 and enter the escape arm after a washout period of ≥ 5 half-lives of the systemic treatment.
Outcomes
This article reports PROs including the percentage of patients achieving ≥ 4-point improvement from baseline to Week 16 on the Skin Pain Numeric Rating Scale (NRS) (a daily, participant-administered, 11-point horizontal scale anchored at 0 and 10 (0 = “no pain”, 10 = “worst pain imaginable”) [22]); change from baseline to Weeks 4, 8, 12, and 16 on the Dermatology Life Quality Index (DLQI) and the Children’s DLQI (CDLQI) (measuring quality of life [23]); change from baseline to Week 16 on the Patient Oriented Eczema Measure (POEM; measuring the patient interpretation of disease severity, and frequency of 7 symptoms [itch, sleep disturbance, dryness, flaking, weeping or oozing, bleeding, and cracking] in the past 7 days [24]); change from baseline to Week 16 in the Work Productivity and Activity Impairment-AD (WPAI-AD) scores for absenteeism, presenteeism, work impairment, and daily activity impairment (evaluating the impact of AD on productivity during the past 7 days, ranging from 0–100 with higher scores indicating greater impairment and less productivity) (25, 26); and the percentage change from baseline to Week 16 in SCORing Atopic Dermatitis (SCORAD; measuring the severity of AD) (27). DLQI assessment was completed by patients > 16 years old in the study clinic and patients ≤ 16 years old used the CDLQI.
Statistical analysis
The sample size was 286 patients, including 18 adolescents, based on enrolment feasibility in Japan, which was determined according to assumed IGA (0,1) and EASI 75 response rates at Week 16. The study had >95% and >80% power to test the superiority of lebrikizumab Q2W + TCS and Q4W + TCS, respectively, vs PBO + TCS in the coprimary endpoints (IGA (0,1) and EASI 75 response rates at Week 16), based on a 2-sided Fisher exact test with α = 0.05. All PRO endpoints were analysed in the intent-to-treat (ITT) population, defined as all randomized patients. No multiplicity adjustment was employed in the analysis for the PRO endpoints reported in this manuscript. Binary PRO endpoints were analysed using non-responder imputation. Binary endpoints were analysed using the Cochran–Mantel–Haenszel (CMH) test adjusted for the stratification factors (age [adolescent patients 12 to < 18 years versus adults ≥ 18 years] and disease severity [IGA 3 versus 4]). Continuous endpoints were analysed using a mixed-effects model for repeated measures, using treatment, baseline value, visit, baseline-by-visit interactions, treatment-by-visit interactions, and stratification factors as fixed effects. Treatment difference was evaluated by calculating the common risk difference and its 95% confidence interval (CI) and CMH p-value for binary endpoints, and by least squares mean (LSM) difference, standard error (SE), 95% CI, and p-value for continuous endpoints. Statistical analyses were performed using SAS Version 9.4 (SAS Institute Inc, Cary, NC, USA).
RESULTS
Baseline characteristics
Of the 320 patients screened, 286 patients, including 18 adolescents, were randomized 2:2:3 to receive placebo (82 patients), lebrikizumab Q4W (81 patients), and lebrikizumab Q2W (123 patients) in combination with TCS, received ≥ 1 dose of study intervention, and were included in the ITT population. A total of 282 patients (98.6%) completed the 16-week induction period (PBO + TCS: 82 patients; LEBQ4W + TCS: 80 patients; LEBQ2W + TCS: 120 patients). Four (1.4%) patients discontinued the study due to AEs (LEBQ2W + TCS: 2 patients) and patient withdrawal (LEBQ4W + TCS and LEBQ2W + TCS: 1 patient each). Baseline demographics and patient characteristics were similar in all 3 treatment groups (Table I). The mean (standard deviation [SD]) age of patients was 34.8 (13.6) (PBO + TCS), 37.8 (12.0) (LEBQ4W + TCS), and 35.5 years (12.2) (LEBQ2W + TCS). The mean (SD) duration of AD was 29.0 (14.1) years in PBO + TCS, 28.7 (13.7) in LEBQ4W + TCS, and 28.2 (13.1) in LEBQ2W + TCS. At baseline, 32.9% of patients in PBO + TCS, 32.1% in LEBQ4W + TCS, and 31.7% of patients in LEBQ2W + TCS had severe AD, based on IGA score.
| Variable | Placebo + TCS (n = 82) | Lebrikizumab Q4W + TCS (n = 81) | Lebrikizumab Q2W + TCS (n = 123) |
| Age, years, mean (SD)a | 34.8 (13.6) | 37.8 (12.0) | 35.5 (12.2) |
| Adults (≥ 18 years) | 76 (92.7) | 77 (95.1) | 115 (93.5) |
| Adolescents (≥ 12 to < 18 years) | 6 (7.3) | 4 (4.9) | 8 (6.5) |
| Female, n (%) | 24 (29.3) | 25 (30.9) | 41 (33.3) |
| Asian, n (%) | 82 (100) | 81 (100) | 123 (100) |
| Duration of AD, years, mean (SD) | 29.0 (14.1) | 28.7 (13.7) | 28.2 (13.1) |
| Weight, kg, mean (SD) | 65.6 (12.5) | 66.5 (13.8) | 64.9 (11.4) |
| < 60 kg | 29 (35.4) | 29 (35.8) | 47 (38.2) |
| ≥ 60 to < 100 kg | 53 (64.6) | 51 (63.0) | 76 (61.8) |
| ≥ 100 kg | 0 | 1 (1.2) | 0 |
| BMI, kg/m2, mean (SD) | 23.6 (3.8) | 23.7 (3. 9) | 23.6 (3.8) |
| Prespecified medical history, n (%) | |||
| Hand dermatitis | 24 (29.3) | 16 (19.8) | 28 (22.8) |
| Facial dermatitis | 18 (22.0) | 13 (16.0) | 23 (18.7) |
| Conjunctivitis | 26 (31.7) | 27 (33.3) | 41 (33.3) |
| Herpes zoster | 17 (20.7) | 17 (21.0) | 22 (17.9) |
| Chickenpox | 37 (45.1) | 42 (51.9) | 51 (41.5) |
| Eczema herpeticum | 10 (12.2) | 5 (6.2) | 12 (9.8) |
| Herpetic whitlow | 2 (2.4) | 0 | 1 (0.8) |
| Disseminated neonatal herpes simplex | 0 | 0 | 4 (3.3) |
| Herpes labialis | 14 (17.1) | 18 (22.2) | 19 (15.4) |
| Genital herpes | 2 (2.4) | 2 (2.5) | 0 |
| Asthma | 25 (30.5) | 22 (27.2) | 41 (33.3) |
| Alopecia | 7 (8.5) | 3 (3.7) | 13 (10.6) |
| Food allergy | 22 (26.8) | 23 (28.4) | 39 (31.7) |
| Allergic rhinitis | 35 (42.7) | 35 (43.2) | 66 (53.7) |
| Pollinosis | 47 (57.3) | 38 (46.9) | 69 (56.1) |
| Prior AD treatment, n (%) | |||
| Topical corticosteroids | 75 (91.5) | 76 (93.8) | 115 (93.5) |
| Topical calcineurin inhibitors | 40 (48.8) | 33 (40.7) | 50 (40.7) |
| Topical JAK inhibitors | 19 (23.2) | 22 (27.2) | 29 (23.6) |
| Topical PDE-4 inhibitors | 0 | 0 | 0 |
| Systemic corticosteroids | 35 (42.7) | 29 (35.8) | 42 (34.1) |
| Immunosuppressants | 13 (15.9) | 13 (16.0) | 19 (15.4) |
| Biologics | 2 (2.4) | 3 (3.7) | 2 (1.6) |
| Phototherapy | 11 (13.4) | 17 (21.0) | 21 (17.1) |
| IGA score | |||
| 3 (moderate) | 55 (67.1) | 55 (67.9) | 84 (68.3) |
| 4 (severe) | 27 (32.9) | 26 (32.1) | 39 (31.7) |
| EASI, mean (SD) | 33.7 (11.8) | 34.2 (13.0) | 32.0 (11.5) |
| SCORAD, mean (SD) | 63.2 (12.1) | 65.8 (13.4) | 61.8 (13.6) |
| BSA % affected, mean (SD) | 60.6 (18.4) | 59.6 (20.7) | 58.4 (18.2) |
| Pruritus NRS, mean (SD) | 5.2 (1.9) | 5.4 (2.1) | 5.1 (2.2) |
| ≥ 4 | 60 (74.1) | 59 (72.8) | 80 (65.0) |
| Sleep-loss due to itch, mean (SD) | 1.5 (0.9) | 1.5 (0.9) | 1.4 (0.8) |
| ≥ 2 | 26 (32.1) | 26 (32.1) | 36 (29.3) |
| Skin Pain NRS, mean (SD) | 4.1 (2.3) | 4.2 (2.7) | 4.0 (2.5) |
| POEM, mean (SD) | 17.0 (7.4) | 16.7 (7.1) | 15.9 (6.8) |
| DLQI, mean (SD) | 8.1 (5.3) | 8.4 (5.6) | 8.3 (4.9) |
| CDLQI, mean (SD) | 5.4 (1.8) | 9.0 (2.9) | 7.4 (5.2) |
| WPAI-AD Employment Status, n (%) | |||
| Employed | 65 (79.3) | 70 (86.4) | 95 (77.2) |
| Not employed | 17 (20.7) | 11 (13.6) | 28 (22.8) |
| WPAI-AD Absenteeism, % mean | 1.5 (12.4) | 0.4 (2.4) | 1.5 (7.1) |
| WPAI-AD Presenteeism, % mean | 31.6 (26.0) | 34.6 (29.6) | 36.0 (28.1) |
| WPAI-AD Overall work impairment, % mean | 31.6 (26.0) | 34.9 (29.6) | 36.4 (28.5) |
| WPAI-AD Activity Impairment, % mean | 36.5 (25.2) | 43.8 (29.9) | 40.2 (27.7) |
| aAge was calculated at the informed consent date. Birth date of adult patients and adolescent patients was imputed as 1 July in the reported birth year and as the 15th day in the reported birth month, respectively. | |||
| AD: atopic dermatitis; BMI: body mass index; BSA: body surface area; CDLQI: Children’s Dermatology Life Quality Index; EASI: Eczema Area and Severity Index; IGA: Investigator’s Global Assessment; ITT: intent-to-treat; JAK: Janus kinase; NRS: numeric rating scale; PDE-4: phosphodiesterase-4; POEM: Patient Oriented Eczema Measure; Q2W: every 2 weeks; Q4W: every 4 weeks; SCORAD: SCORing Atopic Dermatitis; SD: standard deviation; WPAI-AD: Work Productivity and Activity Impairment – Atopic Dermatitis. | |||
Skin Pain Numeric Rating Scale
Lebrikizumab in combination with TCS provided significant improvements in skin pain at Week 16 compared with placebo in combination with TCS, as measured by the proportion of patients reporting a score of ≥ 4 at baseline who achieved a ≥ 4-point improvement from baseline in Skin Pain NRS. At Week 16, a significantly greater proportion of patients receiving LEBQ4W + TCS (35.0%, p < 0.001) or receiving LEBQ2W + TCS (48.4%, p < 0.001) achieved a Skin Pain NRS ≥ 4-point improvement from baseline compared with PBO + TCS (4.7%). Significance was achieved at Week 4 for both LEBQ4W + TCS (p < 0.01) and LEBQ2W + TCS (p < 0.001, Fig. 1).
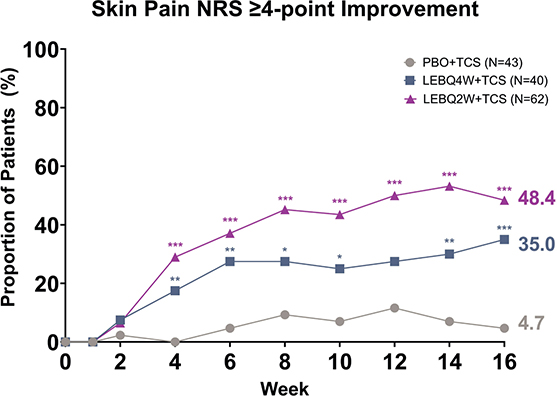
Fig. 1. Time Course Response for Skin Pain ≥ 4-point improvement from baseline. Percentage of patients (%) reporting a Skin Pain Numeric Rating Scale (NRS) score of ≥ 4 points at baseline achieving a ≥ 4-point improvement from baseline. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 vs placebo in combination with topical corticosteroids (TCS) using the Cochran–Mantel–Haenszel test with non-responder imputation. LEB: lebrikizumab; N: number of patients in the analysis population; PBO: placebo; Q2W: every 2 weeks; Q4W: every 4 weeks.
Dermatology Life Quality Index/Children’s Dermatology Life Quality Index
Both LEB + TCS treatment groups had statistically significant improvements compared with the PBO + TCS group in DLQI at Week 16. Significant improvements in DLQI score change from baseline were seen in LEBQ4W + TCS (–3.2, p < 0.001) and LEBQ2W + TCS (–4.6, p < 0.001) compared with PBO + TCS (–0.1). Significance was achieved at Weeks 4, 8, 12, and 16 for both LEBQ4W + TCS (p < 0.001) and LEBQ2W + TCS (p < 0.001, Fig. 2A). Numerical differences were observed in CDLQI change from baseline scores in LEBQ4W + TCS (–3.5) and LEBQ2W + TCS (–3.5) compared with PBO + TCS (–2.1) (Fig. 2B).
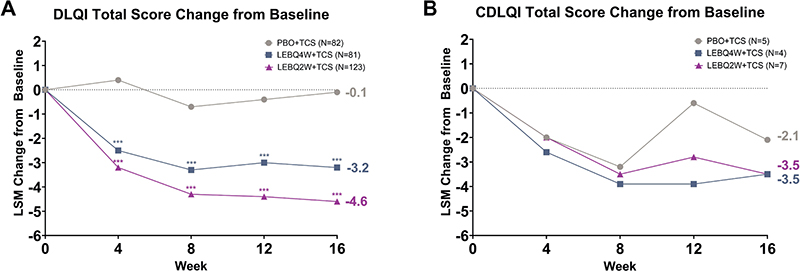
Fig. 2. Dermatology Life Quality Index (DLQI) and Children’s DLQI (CDLQI) total score change from baseline. Time course response from baseline to Week 16 for DLQI (A) and CDLQI (B) total scores. DLQI was measured starting at Week 4. DLQI assessment was completed by patients > 16 years old and patients ≤ 16 years old used the CDLQI. ***p < 0.001 vs placebo in combination with topical corticosteroids (TCS) using mixed model repeated measures. LEB: lebrikizumab; LSM: least squares mean; N: number of patients in the analysis population; PBO: placebo; Q2W: every 2 weeks; Q4W: every 4 weeks.
Patient Oriented Eczema Measure
The mean (SD) baseline POEM scores were 17.0 (7.4) for PBO + TCS, 16.7 (7.1) for LEBQ4W + TCS, and 15.9 (6.8) for LEBQ2W + TCS. The change in POEM from baseline to Week 16 was similar between the 2 LEB + TCS groups and statistically significant compared with PBO + TCS (p < 0.001). The POEM total score change from baseline was –6.8 (LEBQ4W + TCS), –7.9 (LEBQ2W + TCS), and –0.3 (PBO + TCS). Significance was achieved as early as Week 2 for LEBQ4W + TCS (p < 0.01) and LEBQ2W + TCS (p < 0.001) and was maintained at Weeks 4 to 16 for both LEBQ4W + TCS (p < 0.001) and LEBQ2W + TCS (p < 0.001, Fig. 3).
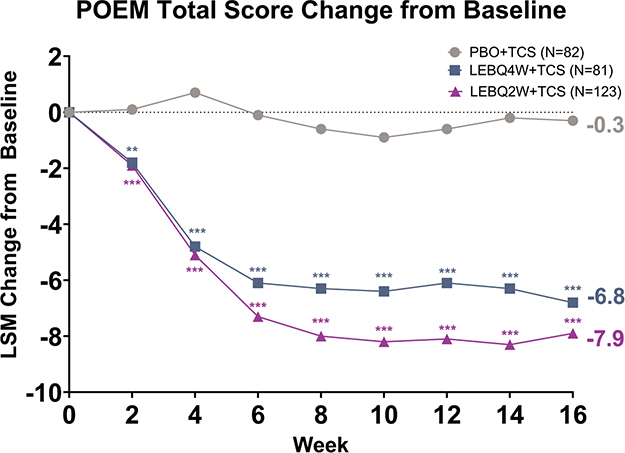
Fig. 3. Patient Oriented Eczema Measure (POEM) least squares mean (LSM) change from baseline. Time course response from baseline to Week 16 for POEM. **p < 0.01, ***p < 0.001 vs placebo in combination with TCS using mixed-model repeated measures. LEB: lebrikizumab; N: number of patients in the analysis population; PBO: placebo; Q2W: every 2 weeks; Q4W: every 4 weeks; TCS: topical corticosteroids.
Work Productivity and Activity Impairment-Atopic Dermatitis
Statistically significant improvements in 3 out of the 4 WPAI-AD scales were observed in both LEB + TCS treatment groups compared with PBO + TCS (Fig. 4). The LSM change from baseline for the WPAI-AD absenteeism scale was –0.9 (LEBQ4W + TCS), –0.6 (LEBQ2W + TCS), and –0.6 (PBO + TCS). Significant differences were observed in the presenteeism scale in both LEBQ4W + TCS (–16.2) and LEBQ2W + TCS (–21.5) compared with PBO + TCS (–2.1, p < 0.001). Significant differences were observed in the overall work impairment score in both LEBQ4W + TCS (–16.5) and LEBQ2W + TCS (–21.7) compared with PBO + TCS (–2.2, p < 0.001). Statistical differences were also observed in the percentage impairment in activities performed outside of work in both LEBQ4W + TCS (–17.5) and LEBQ2W + TCS (–24.1) compared with PBO + TCS (–2.3, p < 0.001).
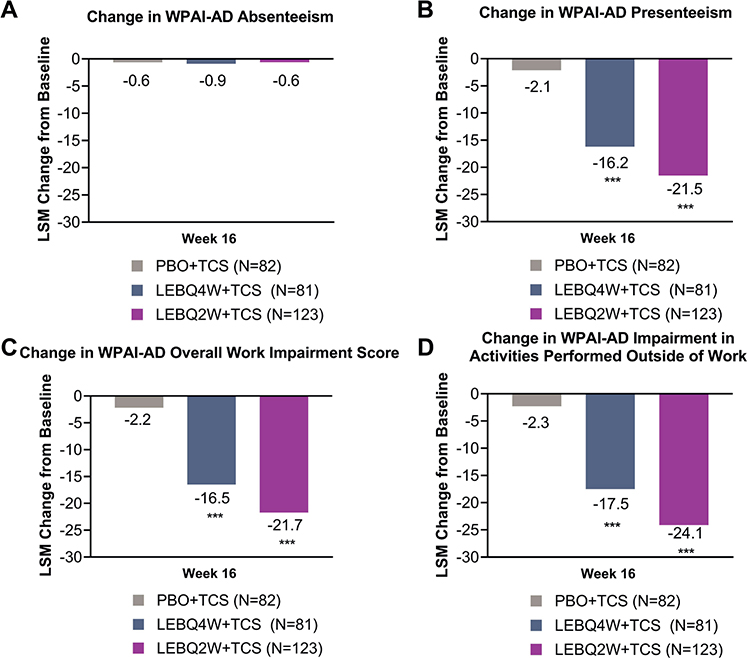
Fig. 4. Change in Work Productivity and Activity Impairment – Atopic Dermatitis (WPAI-AD) score from baseline to week 16. Change in (A) WPAI-AD absenteeism, (B) presenteeism, (C) overall work impairment, and (D) impairment in activities performed outside of work scores from baseline to Week 16. ***p < 0.001 vs placebo in combination with topical corticosteroids (TCS) using mixed-model repeated measures. LEB: lebrikizumab; LSM: least squares mean; N: number of patients in the analysis population; PBO: placebo; Q2W: every 2 weeks; Q4W: every 4 weeks.
SCORing Atopic Dermatitis
Statistically significant improvements in SCORAD were observed in both LEB + TCS treatment groups compared with PBO + TCS. At Week 16, significant improvements in SCORAD percentage change from baseline were seen in LEBQ4W + TCS (–50.9%, p < 0.001) and LEBQ2W + TCS (–55.7%, p < 0.001) compared with PBO + TCS (–21.1%). Significance was achieved as early as Week 2 for both LEB + TCS groups (p < 0.001) and was maintained at Weeks 4 through 16 for both LEBQ4W + TCS (p < 0.001) and LEBQ2W + TCS (p < 0.001, Fig. 5).
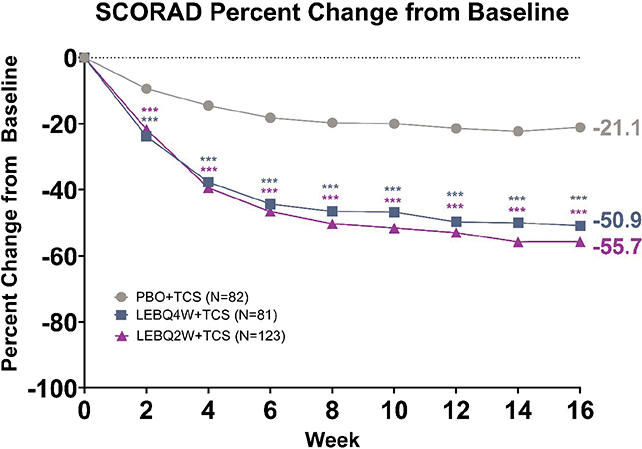
Fig. 5. SCORing Atopic Dermatitis (SCORAD) percentage change from baseline. Percentage change from baseline to Week 16 for SCORAD. ***p < 0.001 vs placebo in combination with topical corticosteroids (TCS) using mixed-model repeated measures. LEB: lebrikizumab; N: number of patients in the analysis population; PBO: placebo; Q2W: every 2 weeks; Q4W: every 4 weeks.
DISCUSSION
ADhere-J was the first Phase 3 randomized placebo-controlled study to evaluate the efficacy and safety of lebrikizumab combined with TCS in Japanese adults and adolescents with moderate-to-severe AD. In this analysis, lebrikizumab 250 mg Q4W and Q2W in combination with TCS improved PROs in Japanese patients with -moderate-to-severe AD up to 16 weeks. All PRO endpoints for the study were met; patients in the lebrikizumab in combination with TCS treatment groups demonstrated statistically significant and clinically meaningful improvements compared with placebo in combination with TCS on Skin Pain NRS, DLQI, POEM, WPAI-AD, and SCORAD scales.
In this manuscript, itch was assessed as part of the DLQI, POEM, and SCORAD scales. Itch is a predominant feature of AD and can have a substantial impact on overall AD burden, quality of life, work productivity, sleep, emotional state, and daily activities (6, 7, 28–30). A real-world evidence study drew data from the 2020 Adelphi AD Disease Specific Program to describe the burden of itch and skin pain in adult patients with AD in Japan (26). Patients experiencing itch and skin pain had significantly worse POEM scores, more overall work impairment, sleep disruption, higher EASI scores, and more areas affected compared with those without itch or skin pain. A study presenting the burden estimate for AD using data from the Global Burden of Disease Study found that AD ranks 15th among all nonfatal diseases and has the highest disease burden among skin diseases as measured by disability-adjusted life-years (31). This demonstrates the need for better treatments to combat these aspects of AD. In the present study, lebrikizumab Q2W + TCS significantly improved itch compared with PBO + TCS as early as Week 2, and lebrikizumab Q4W + TCS significantly improved itch from Week 4, indicating that, in addition to skin improvement, lebrikizumab is effective in providing itch relief in Japanese patients with moderate-to-severe AD. This is consistent with the global ADvocate and ADhere studies, where lebrikizumab monotherapy and in combination with TCS improved quality of life through improvement in itch and interference of itch on sleep (32). Lebrikizumab has also shown a durable reduction in the interference of itch on sleep through to 52 weeks in patients with moderate-to-severe AD who were responders to lebrikizumab at the end of the 16-week induction period (33). Sustained improvements in itch interference on sleep were observed for Week 16 responders across all treatment arms, including the withdrawal arm, indicating durability of response to lebrikizumab treatment.
Skin pain is common in patients with AD, with up to 61% of patients reporting this symptom and 14% describing the pain as severe or very severe (34). Furthermore, a recent survey of dermatologists and patients with a history of moderate or severe AD investigating the impact of both itch and skin pain on quality of life and treatment satisfaction in Japan demonstrated the additional burden associated with both itch and skin pain compared with itch alone (26). Key findings of the study were that itch and skin pain were associated with greater clinical severity in terms of body region and BSA affected, greater dissatisfaction with disease control and, from the patient perspective, greater sleep disruption, work impairment, and poorer quality of life. ADhere-J is the first study to report the impact of lebrikizumab on skin pain and showed that treatment with lebrikizumab in combination with TCS improved skin pain, with 35–48% of lebrikizumab + TCS treated patients achieving ≥ 4-point improvement. Significant differences compared with placebo were observed from Week 4 for both lebrikizumab + TCS treatment groups. The implications of these results are important due to the impact that the symptom of skin pain in AD has on quality of life, including negative impacts on mental health, sleep, socializing, and daily life (5, 35). A decrease of 33% has been identified as a clinically meaningful change in pain from the patient’s perspective (36). The impact of lebrikizumab treatment in combination with TCS on work productivity was evaluated using WPAI-AD, and the analysis showed statistically significant improvements in 3 out of the 4 WPAI-AD scores in both lebrikizumab + TCS groups compared with PBO + TCS (presenteeism, overall work impairment, and impairment in activities performed outside of work).
POEM was identified as the core instrument to measure symptoms by the Harmonizing Outcome Measures for Eczema (HOME) initiative (37). Severity strata or bands were developed to improve interpretability and determination of clinical meaningfulness. The proposed bandings for POEM scores are 0–2 (clear/almost clear); 3–7 (mild); 8–16 (moderate); 17–24 (severe); and 25–28 (very severe) (25). A significant improvement in POEM was achieved as early as Week 2 and was maintained at Weeks 4 to 16 for both lebrikizumab + TCS doses.
Significant improvements in SCORAD were also seen from Week 2 for both lebrikizumab + TCS treatment groups and met the meaningful minimal important change (MIC) for SCORAD of 35% (38).
In the global ADhere TCS combination study, significant improvements were observed in itch, DLQI, and sleep loss due to itch (18). A higher proportion of patients receiving lebrikizumab in combination with TCS achieved at least a 4-point reduction in Pruritus NRS score and in DLQI score at Week 16 compared with those receiving placebo plus TCS. Furthermore, there was a significant difference in Sleep-Loss Scale score change from baseline at Week 16. In comparison with the global ADhere study, TCS in ADhere-J was not washed out before randomization, suggesting that the treatment effect of lebrikizumab combined with TCS was clearly reflected in the results. The difference in the type of low- and mid-potency TCS used in this study versus ADhere may have also affected response rates. Of the lebrikizumab+TCS treated patients in ADhere-J, 53–69% achieved ≥4-point improvement, or DLQI <4 (minimal disease impact).
Strengths and limitations
Strengths of the ADhere-J study included the randomized, double-blind, placebo-controlled, parallel-group study design. This study also had a sufficient sample size to test the superiority of lebrikizumab + TCS over PBO + TCS using various PRO endpoints and the study discontinuation rate was low. Furthermore, patients in ADhere-J had no washout period and started TCS at least 7 days before baseline, which is similar to how advanced biologics might be used in clinical settings. Consequently, a limited number of patients required rescue treatment. Finally, this study provided long-term evaluation of lebrikizumab + TCS efficacy and safety up to 68 weeks.
The limitations of this study included strict eligibility criteria that may limit generalizability of results, and the small number of adolescent patients in the study, which impacted the CDLQI analysis.
In conclusion, results from this study in Japanese patients with moderate-to-severe AD demonstrated that lebrikizumab combined with TCS compared with placebo with TCS produced early and sustained improvements up to Week 16 in PROs. These PRO improvements in Skin Pain NRS, DLQI, POEM, WPAI-AD, and SCORAD scales support lebrikizumab as a promising treatment option in this population.
ACKNOWLEDGEMENTS
Role of the funder/sponsor: Eli Lilly Japan K.K. was involved in the study design, data collection, data analysis, and preparation of the manuscript. Almirall, S.A. has licensed the rights to develop and commercialize lebrikizumab for the treatment of dermatology indications, including atopic dermatitis in Europe. Eli Lilly and Company has exclusive rights for development and commercialization of lebrikizumab in the United States and the rest of the world outside of Europe.
Additional contributions/acknowledgements: The authors would like to thank all study participants and the following study investigators: Masatoshi Abe from Sapporo Skin Clinic, Nobuhiro Fujita from Sumire Dermatology Clinic, Yasushi Goto from Goto Dermatology Clinic, Hideki Ito from Tachikawa Dermatology Clinic, Hiroki Kanda from Mita Dermatology Clinic, Junko Kawashima from Kawashima Dermatology, Yumiko Kudo from Tanpopo Dermatology Clinic, Akihiro Kume from Dermatology and Ophthalmology Kume Clinic, Kazunobu Mochida from Mochida Dermatology Clinic, Juichiro Nakayama from Jozan Dermatology-Urology Clinic, Hiromitsu Noguchi from Noguchi Dermatology Clinic, Yuko Nomura from Nomura Dermatology Clinic, Naoki Sakurai from Charme Clinique, Aisaku Yamamoto from Kosugi Dermatology Clinic, Yasuaki Yanagihara from Yanagihara Dermatology Clinic, Kazutomo Toyofuku from Yamate Dermatological Clinic, Reiko Yoshimura from Medical Corporation H.S.C., Satsuki Kubota from Sanritsu Dermatology Clinic, Yasuhiro Kawachi from Tokyo Medical University Ibaraki Medical Center, Chiharu Tateishi from Osaka City University Hospital and Osaka Metropolitan University Hospital, Yuuki Horiuchi from Akihabara Skin Clinic, Kenzo Kaji from Kaji Dermatology Clinic, Hitoshi Kobayashi from Kobayashi Skin Clinic, Ryuji Maruyama from Maruyama Dermatology Clinic, Tomoko Matsuda from Matsuda Tomoko Dermatological Clinic, Tokuya Omi from Queen’s Square Medical Center, Fumiaki Shirasaki from Shirasaki Dermatology Clinic, Hideyasu Takata from Sanrui Dermatology Clinic, Shinichiro Yasumoto from Yasumoto Dermatology Clinic, Nobuko Yoshihara from Yoshihara Dermatology Clinic, Takeshi Yoshikawa from Yoshikawa Dermatology Clinic, and Ryoshuke Hino from Hino Dermatology Clinic.
Data sharing statement: Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the United States and European Union and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, and blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
REFERENCES
- Tanaka T, et al. Prevalence of dermatological disorders in Japan: a nationwide, cross-sectional, seasonal, multicenter, hospital-based study. J Dermatol 2011; 38: 310–320. https://doi.org/10.1111/j.1346-8138.2011.01209.x
- Katoh N, Saeki H, Kataoka Y, Etoh T, Teramukai S, Takagi H, et al. Atopic dermatitis disease registry in Japanese adult patients with moderate to severe atopic dermatitis (ADDRESS-J): baseline characteristics, treatment history and disease burden. J Dermatol 2019; 46: 290–300. https://doi.org/10.1111/1346-8138.14787
- Saeki H, Iizuka H, Mori Y, Akasaka T, Takagi H, Kitajima Y, et al. Prevalence of atopic dermatitis in Japanese elementary schoolchildren. Br J Dermatol 2005; 152: 110–114. https://doi.org/10.1111/j.1365-2133.2004.06271.x
- Saeki H, Tsunemi Y, Fujita H, Kagami S, Sasaki K, Ohmatsu H, et al. Prevalence of atopic dermatitis determined by clinical examination in Japanese adults. J Dermatol 2006; 33: 817–819. https://doi.org/10.1111/j.1346-8138.2006.00187.x
- Vakharia PP, Chopra R, Sacotte R, Patel KR, Singam V, Patel N, et al. Burden of skin pain in atopic dermatitis. Ann Allergy Asthma Immunol 2017; 119: 548–552. https://doi.org/10.1016/j.anai.2017.09.076
- Silverberg JI, Gelfand JM, Margolis DJ, Boguniewicz M, Fonacier L, Grayson MH, et al. Patient burden and quality of life in atopic dermatitis in US adults: a population-based cross-sectional study. Ann Allergy Asthma Immunol 2018; 121: 340–347. https://doi.org/10.1016/j.anai.2018.07.006
- Chrostowska-Plak D, Reich A, Szepietowski JC. Relationship between itch and psychological status of patients with atopic dermatitis. J Eur Acad Dermatol Venereol 2013; 27: e239–e242. https://doi.org/10.1111/j.1468-3083.2012.04578.x
- Yano C, Saeki H, Ishiji T, Ishiuji Y, Sato J, Tofuku Y, et al. Impact of disease severity on work productivity and activity impairment in Japanese patients with atopic dermatitis. J Dermatol 2013; 40: 736–739. https://doi.org/10.1111/1346-8138.12220
- Puar N, Chovatiya R, Paller AS. New treatments in atopic dermatitis. Ann Allergy Asthma Immunol 2021; 126: 21–31. https://doi.org/10.1016/j.anai.2020.08.016
- Dhadwal G, Albrecht L, Gniadecki R, Poulin Y, Yeung J, Hong C-h, et al. Approach to the assessment and management of adult patients with atopic dermatitis: a consensus document. Section IV: treatment options for the management of atopic dermatitis. J Cutan Med Surg 2018; 22: 21S–29S. https://doi.org/10.1177/1203475418805721
- Kamei K, Hirose T, Yoshii N, Tanaka A. Burden of illness, medication adherence, and unmet medical needs in Japanese patients with atopic dermatitis: a retrospective analysis of a cross-sectional questionnaire survey. J Dermatol 2021; 48: 1491–1498. https://doi.org/10.1111/1346-8138.16054
- Kim J, Kim BE, Leung DYM. Pathophysiology of atopic dermatitis: clinical implications. Allergy Asthma Proc 2019; 40: 84–92. https://doi.org/10.2500/aap.2019.40.4202
- Tsoi LC, Rodriguez E, Degenhardt F, Baurecht H, Wehkamp U, Volks N, et al. Atopic dermatitis is an IL-13-dominant disease with greater molecular heterogeneity compared to psoriasis. J Invest Dermatol 2019; 139: 1480–1489. https://doi.org/10.1016/j.jid.2018.12.018
- Bieber T. Interleukin-13: targeting an underestimated cytokine in atopic dermatitis. Allergy 2020; 75: 54–62. https://doi.org/10.1111/all.13954
- Szegedi K, Lutter R, Bos JD, Luiten RM, Kezic S, Middelkamp-Hup MA. Cytokine profiles in interstitial fluid from chronic atopic dermatitis skin. J Eur Acad Dermatol Venereol 2015; 29: 2136–2144. https://doi.org/10.1111/jdv.13160
- Okragly A, Ryuzoji A, Daniels M, Patel C, Benschop R. Comparison of the affinity and in vitro activity of lebrikizumab, tralokinumab, and cendakimab. 4th Inflammatory Skin Disease Summit, New York, November 3–6, 2021. Exp Dermatol 2021; 30: 3–43.
- Silverberg JI, Guttman-Yassky E, Thaçi D, Irvine AD, Stein Gold L, Blauvelt A, et al. Two phase 3 trials of lebrikizumab for moderate-to-severe atopic dermatitis. N Engl J Med 2023; 388: 1080–1091. https://doi.org/10.1056/NEJMoa2206714
- Simpson EL, Gooderham M, Wollenberg A, Weidinger S, Armstrong A, Soung J, et al. Efficacy and safety of lebrikizumab in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis: a randomized clinical trial (ADhere). JAMA Dermatol 2023; 159: 182–191. https://doi.org/10.1001/jamadermatol.2022.5534
- Eichenfield LF, Tom WL, Berger TG, Krol A, Paller AS, Schwarzenberger K, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol 2014; 71: 116–132. https://doi.org/10.1016/j.jaad.2014.03.023
- Hanifin JM, Thurston M, Omoto M, Cherill R, Tofte SJ, Graeber M, et al. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. Exp Dermatol 2001; 10: 11–18. https://doi.org/10.1034/j.1600-0625.2001.100102.x
- Simpson E, Bissonnette R, Eichenfield LF, Guttman-Yassky E, King B, Silverberg JI, et al. The Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD): the development and reliability testing of a novel clinical outcome measurement instrument for the severity of atopic dermatitis. J Am Acad Dermatol 2020; 83: 839–846. https://doi.org/10.1016/j.jaad.2020.04.104
- Silverberg JI, DeLozier A, Sun L, Thyssen JP, Kim B, Yosipovitch G, et al. Psychometric properties of the itch numeric rating scale, skin pain numeric rating scale, and atopic dermatitis sleep scale in adult patients with moderate-to-severe atopic dermatitis. Health Qual Life Outcomes 2021; 19: 247. https://doi.org/10.1186/s12955-021-01877-8
- Basra MKA, Salek MS, Camilleri L, Sturkey R, Finlay AY. Determining the minimal clinically important difference and responsiveness of the Dermatology Life Quality Index (DLQI): further data. Dermatology 2015; 230: 27–33. https://doi.org/10.1159/000365390
- Charman CR, Venn AJ, Ravenscroft JC, Williams HC. Translating Patient-Oriented Eczema Measure (POEM) scores into clinical practice by suggesting severity strata derived using anchor-based methods. Br J Dermatol 2013; 169: 1326–1332. https://doi.org/10.1111/bjd.12590
- Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics 1993; 4: 353–365. https://doi.org/10.2165/00019053-199304050-00006
- Torisu-Itakura H, Anderson P, Piercy J, Pike J, Sakamoto A, Kabashima K. Impact of itch and skin pain on quality of life in adult patients with atopic dermatitis in Japan: results from a real-world, point-in-time, survey of physicians and patients. Curr Med Res Opin 2022; 38: 1401–1410. https://doi.org/10.1080/03007995.2022.2092352
- Schram ME, Spuls PI, Leeflang MMG, Lindeboom R, Bos JD, Schmitt J. EASI, (objective) SCORAD and POEM for atopic eczema: responsiveness and minimal clinically important difference. Allergy 2012; 67: 99–106. https://doi.org/10.1111/j.1398-9995.2011.02719.x
- Legat FJ. Itch in atopic dermatitis: what is new? Front Med 2021; 8: 644760. https://doi.org/10.3389/fmed.2021.644760
- Murota H, Koike Y, Ishii K, Calimlim BM, Ludwikowska M, Toumi M, et al. Evaluating the burden of pruritus due to atopic dermatitis in Japan by patient-reported outcomes. J Med Econ 2021; 24: 1280–1289. https://doi.org/10.1080/13696998.2021.2002559
- Elmariah SB. Adjunctive management of itch in atopic dermatitis. Dermatol Clin 2017; 35: 373–394. https://doi.org/10.1016/j.det.2017.02.011
- Laughter MR, Maymone MBC, Mashayekhi S, Arents BWM, Karimkhani C, Langan SM, et al. The global burden of atopic dermatitis: lessons from the Global Burden of Disease Study 1990–2017. Br J Dermatol 2021; 184: 304–309. https://doi.org/10.1111/bjd.19580
- Yosipovitch G. Lebrikizumab monotherapy improves quality of life through improvement in itch and sleep: results from two phase 3 trials in patients with moderate-to-severe atopic dermatitis. American Academy of Dermatology – 81st Annual Meeting 2023. https://doi.org/10.1016/j.jaad.2023.07.246
- Yosipovitch G. Lebrikizumab reduced interference of itch on sleep at 52 weeks in patients with moderate-to-severe atopic dermatitis: pooled analysis of 2 phase 3 randomized controlled trials. American Academy of Dermatology – 81st Annual Meeting 2023. https://doi.org/10.26226/m.649c5e4156241620f7b6a64c
- Silverberg JI, Gelfand JM, Margolis DJ, Boguniewicz M, Fonacier L, Grayson MH, et al. Pain is a common and burdensome symptom of atopic dermatitis in United States adults. J Allergy Clin Immunol Pract 2019; 7: 2699–2706. https://doi.org/10.1016/j.jaip.2019.05.055
- Maarouf M, Kromenacker B, Capozza KL, Kempton D, Hendricks A, Tran K, et al. Pain and itch are dual burdens in atopic dermatitis. Dermatitis 2018; 29: 278–281. https://doi.org/10.1097/DER.0000000000000406
- Jensen MP, Chen C, Brugger AM. Interpretation of visual analog scale ratings and change scores: a reanalysis of two clinical trials of postoperative pain. J Pain 2003; 4: 407–414. https://doi.org/10.1016/S1526-5900(03)00716-8
- Spuls PI, Gerbens LAA, Simpson E, Apfelbacher CJ, Chalmers JR, Thomas KS, et al. Patient-Oriented Eczema Measure (POEM), a core instrument to measure symptoms in clinical trials: a Harmonising Outcome Measures for Eczema (HOME) statement. Br J Dermatol 2017; 176: 979–984. https://doi.org/10.1111/bjd.15179
- Silverberg JI, Lei D, Yousaf M, Janmohamed SR, Vakharia PP, Chopra R, et al. What are the best endpoints for Eczema Area and Severity Index and Scoring Atopic Dermatitis in clinical practice? A prospective observational study. Br J Dermatol 2021; 184: 888–895. https://doi.org/10.1111/bjd.19457
Footnote
1Hanada T IK, Kataoka Y, Katoh N, Morisaki Y, Shimizu R, et al. Efficacy and safety of lebrikizumab (LEB) combined with TCS in Japanese patients with moderate-to-severe atopic dermatitis phase 3 study (ADhere-J). Japanese Dermatological Association – 87th Tokyo Branch Meeting, 2023.