Asymmetrical periflexural exanthem of childhood (APEC), also known as unilateral laterothoracic exanthem, is a disorder of unknown aetiology that is prevalent in children; however, it has been associated with viral infections (1). We report here the first paediatric case of APEC associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
CASE REPORT
A 2-year-old girl presented with a dermatosis on the trunk and upper and lower right extremities that affected the right anterior hemithorax, right side of the abdomen, anterior side of the right arm in its proximal region, and anterior side of the right thigh in proximal third. The dermatosis was characterized by erythematous macules that progressed to erythematous papules, 0.5 cm in diameter, with a tendency to converge, with an evolution time of 2 weeks, which were asymptomatic (Figs 1 and 2). She did not develop any extracutaneous clinical manifestations. She had a normal complete blood count without eosinophilia, and her ferritin, d-dimer, and C-reactive protein levels were within normal limits. Reverse transcription polymerase chain reaction (RT-PCR) of a sample obtained by nasopharyngeal swab yielded a positive result for SARS-CoV-2, and viral serologies were negative for herpes 6 and 7, adenovirus, influenza, parainfluenza, and parvovirus B19. The patient had not taken any medications or recreational drugs during the past 3 months and had no epidemiological contact with patients with coronavirus disease 2019 (COVID-19). However, she had a history of visiting a public park without wearing a medical-grade mask like surgical mask 3 times a week. Ten days after the onset of her clinical symptoms, the primary caregiver (mother) and 3 cohabiting adults developed respiratory symptoms with a positive RT-PCR result for SARS-CoV-2.
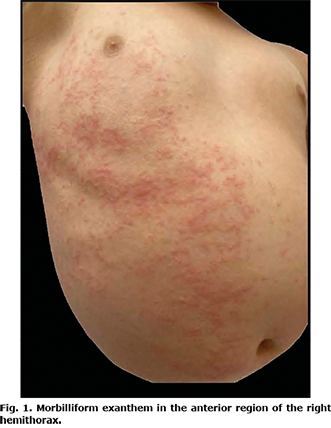
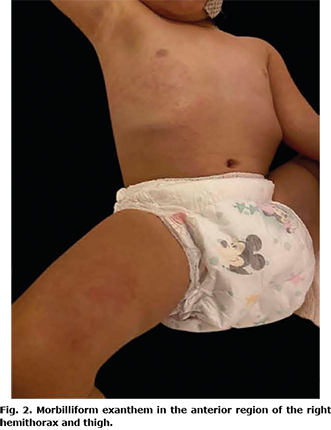
Based on clinical, epidemiological characteristics, and laboratory tests, the final diagnosis was APEC associated with SARS-CoV-2 infection. The patient was the index case in her family. The dermatosis resolved spontaneously after 2 weeks.
DISCUSSION
APEC is a self-limiting disease that is prevalent among children and is characterized by a unilateral eruption of probable viral aetiology. It was first reported in 1962 by Brunner et al. (3) and was designated as “new exanthema” (2, 3).
APEC primarily affects preschool children, although it has also been reported in a 4-month-old infant and in adults (4, 5). It is more prevalent in winter and spring (6). There are limited epidemiological studies of this entity; the mean age of onset is 27.5 months (range 1–5 years) (7). Its origin remains unclear, although the most accepted theory is the viral cause because of the similarity between its onset and course and other viral exanthems, which are generally preceded by symptoms of infection or appear during an eruption. APEC is predominantly seasonal, and small epidemics and family cases have been reported (6, 8). APEC has been associated with parvovirus B19, influenza, parainfluenza 2 and 3, adenovirus, herpes virus 6 and 7, Epstein-Barr virus, poxvirus, rubeola (measles), and Campylobacter jejuni (2, 6, 9–13). According to a systematic review by Chuh et al., more than 300 patients worldwide have been reported in 2016, with most patients having no identifiable viral cause (5). Other associations include co-occurrence with Guillain–Barré syndrome or post-vaccination (6, 11, 12). No association with drug reactions has been described (14).
Since the beginning of the SARS-CoV-2 pandemic, the number of reports of skin manifestations associated with infection has increased. There is only 1 report, of a 42-year-old woman with bilateral periflexural erythema associated with COVID-19; however, there are no reports in paediatric patients (14).
The clinical picture of APEC is characterized by dermatosis that normally begins in the armpit, although it can be in the cubital fossa, inguinal fossa, or popliteal fossa. Subsequently, it centrifugally spreads to a hemibody, with no right or left predominance, and generally affects mucous membranes, face, palms and soles (6, 8). It is characterized by micropapular (even described as scarlatiniform), erythematous rashes that can merge and form large plaques (6, 10). Pruritus is generally mild when it occurs (65%) (7). Certain patients present fever and a small local adenopathy (70%) (7). Koebner’s pseudo-isomorphic phenomenon has also been reported (15). APEC is almost always preceded by an upper respiratory tract infection (66%) (4, 7) and is diagnosed clinically. Biopsy is not required, but could be essential only when a differential diagnosis with other entities is intended or when dealing with an atypical clinical picture (10). In general, serology is negative, as in the case of the current patient. However, the RT-PCR result for SARS-CoV-2 was positive, demonstrating a temporal relationship between exanthem and active infection with this agent.
Differential diagnosis includes other viral exanthems, which were excluded with a negative viral panel; medications or recreational drugs was also eliminated as there was no history of drug use, and eosinophilia was absent. Irritative or allergic contact dermatitis and atopic dermatitis have a different onset and clinical course, showing highly pruritic plaques (6, 7).
Treatment is symptomatic, based on general skin-care measures and, if required, the use of oral antihistamines. Although the use of corticosteroids does not modify the course of the disease, it can contribute to the improvement of pruritus. The prognosis is good, with resolution at 3–6 weeks with mild desquamation (6, 10).
In conclusion, this is the first case report of APEC associated with SARS-COV-2 in a paediatric patient, and demonstrates the importance of making an early diagnosis to establish adequate epidemiological framework and avoid the spread of the disease.
The authors have no conflicts of interest to declare.
REFERENCES
- Taïeb A, Mégraud F, Legrain V, Mortureux P, Maleville, J. Asymmetric periflexural exanthem of childhood. J Am Acad Dermatol 1993; 29: 391–393.
- Cohen-Sors R, Dadban A, Pezron J, Lok C. Asymmetric periflexural exanthem of childhood and influenza virus infection. Dermatol Online J 2020; 26: 13030/qt0qw93417.
- Brunner MJ, Rubin L, Dunlap F. A new papular erythema of childhood. Arch Dermatol 1962; 85: 539–540.
- Chuh A, Zawar V, Sciallis GF, Kepmf W, Lee A. Pityriasis rosea, Gianotti Crosti syndrome, asymmetric periflexural exanthem, papular-purpuric gloves and socks syndrome, eruptive pseudoangiomatosis and eruptive hypomelanosis: do their epidemiological data substantiate infectious etiologies? Infect Dis Rep 2016; 8: 6418.
- Nahm WK, Paiva C, Golomb C, Badiavas E, Laws R. Asymmetric periflexural exanthem of childhood: a case involving a 4-month-old infant. Pediatr Dermatol 2002; 19: 461–462.
- Romero de Ávila Montoya JM, Hueto Najarro A, Barbera Perez PM, Tarraga Marcos MD, Dadlani Dadlani NM, Gonzalez Garcia G. Asymmetric periflexural exanthem of childhood: 2 case reports. Arch Argent Pediatr 2020; 118: 400–404.
- Coustou D, Léauté-Labréze C, Bioulac-Sage P, Labbe L, Taieb A. Asymmetric periflexural exanthem of childhood: a clinical, pathologic, and epidemiologic prospective study. Arch Dermatol 1999; 135: 799–803.
- Gutzmer R, Herbst AR, Kiehl HP, Kapp A, Weiss J. Unilateral laterothoracic exanthem (asymmetrical periflexural exanthem of childhood): report of an adult patient. J Am Acad Dermatol 1997; 37: 484–485.
- Niedermeier A, Pfützner W, Ruzicka T, Thomas P, Happle R. Superimposed lateralized exanthem of childhood: Report of a case related to adenovirus infection. Clin Exp Dermatol 2014; 39: 351–353.
- Navarro-Triviño FJ, Pérez-López I, Ruiz-Villaverde R. Unilateral laterothoracic exanthema. J Pediatr 2021; 229: 306–307.
- McCuaig C, Russo P, Powell J, Pedneault L, Lebel P, Marcoux D. Unilateral laterothoracic exanthem: a clinicopathologic study of forty-eight patients. J Am Acad Dermatol 1996; 34: 979–984.
- Baek YS, Oh CH, Song HJ, Son SB. Asymmetrical periflexural exanthem of childhood with concurrence of molluscum contagiosum infection. Clin Exp Dermatol 2011; 36: 676–677.
- Coustou D, Masquelier B, Lafon ME, Labreze C, Roul S, Bioulac-Sage P, et al. Asymmetric periflexural exanthem of childhood: microbiologic case-control study. Pediatr Dermatol 2000; 17: 169–173.
- Glick LR, Fogel AL, Ramachandran S, Barakat LA. Unilateral laterothoracic exanthem in association with coronavirus disease 2019. JAAD Case Rep 2020; 6: 900–901.
- Dar NR, Raza N. Asymmetrical periflexural exanthem exhibiting pseudoisomorphic Köebner response in an adult. Clin Exp Dermatol 2009; 34: 808–810.