SHORT COMMUNICATION
Fixed Drug Eruption due to Dupilumab
Ayaka KANEOKA, Natsuko SAITO-SASAKI, Yumiko SAKURAGI and Yu SAWADA*
Department of Dermatology, University of Occupational and Environmental Health, 1-1, Iseigaoka, Yahatanishi-ku, Kitakyushu, 807-8555, Japan. *E-mail: long-ago@med.uoeh-u.ac.jp
Citation: Acta Derm Venereol 2024; 104: adv37804. DOI: https://doi.org/10.2340/actadv.v104.37804.
Copyright: © 2024 The Author(s). Published by MJS Publishing, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Submitted: Jan 9, 2024; Accepted after review: Apr 3, 2024; Published: Apr 20, 2024
INTRODUCTION
Drug-induced cutaneous allergic reactions are widely observed with various medications (1). Among the various drug eruptions, fixed drug eruption is a cell-mediated immunologic reaction connected to molecular structure (2), and is a known adverse cutaneous reaction of monoclonal antibodies against human diseases (3). Dupilumab, a humanized monoclonal antibody against the IL-4/IL-13 receptor, has shown high efficacy against atopic dermatitis (4). However, several cutaneous adverse allergic reactions have been reported. Here, we report a case presenting an unusual clinical manifestation: multiple fixed drug eruptions caused by dupilumab.
CASE REPORT
The patient noticed multiple round dark macules on his left arm, and the eruption gradually developed with repeated administration of dupilumab (Fig. 1A, B). He was referred to our department for an evaluation of his skin eruption. The patient initially presented to the otolaryngology department 2 years ago, exhibiting symptoms consistent with eosinophilic sinusitis. Following a comprehensive assessment, dupilumab treatment was initiated 16 months ago, involving the administration of biweekly subcutaneous injections. The patient has a medical history encompassing Parkinson’s disease, cervical spinal stenosis, and constipation. His current pharmacotherapeutic regimen includes oral administration of levodopa/carbidopa hydrate, pregabalin besylate, and magnesium oxide.
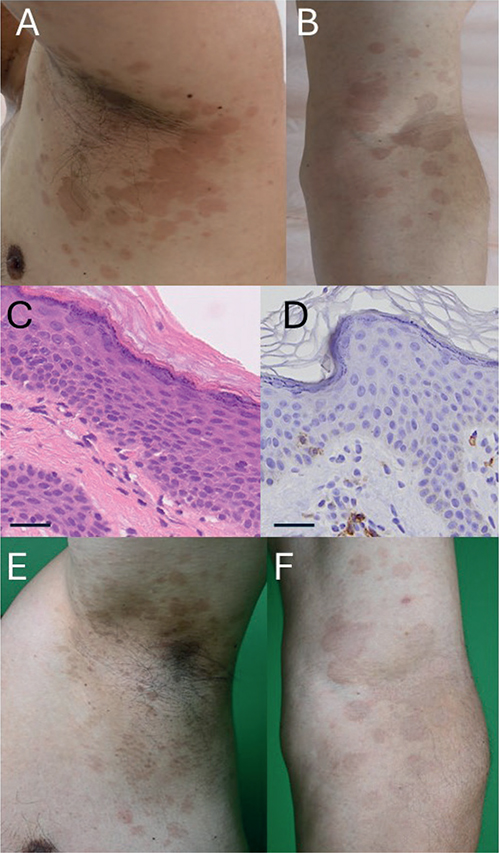
Fig. 1. Clinical and histological images. (A, B) Clinical manifestations at the first visit. Multiple round dark macules on his left axillary region (A) and arm (B). (C) A skin biopsy showed a dyskeratotic keratinocyte in the epidermis and lymphocyte infiltration around vessels in the dermis. Scale bar: 50μm. (D) Immunostaining for CD8. Scale bar: 50μm. (E, F) Clinical manifestations after 3 months. Multiple round macules were impaired on his left axillary region (E) and arm (F).
Treatment with levodopa-carbidopa hydrate for Parkinson’s disease, gabapentin enacarbil for neuropathic pain, and magnesium oxide for constipation was initiated at 1, 3, and 8 months following the onset of the skin eruption, respectively. He did not take any over-the-counter medications, natural/herbal medicines, or dietary supplements during the occurrence of the skin eruption. Physical examination revealed round-shaped dark red macules on his left arm and axillae. A skin biopsy from his left arm showed dyskeratotic keratinocytes in the epidermis and lymphocyte infiltration around vessels in the dermis (Fig. 1C, D). Based on the history, we diagnosed his skin eruption as a multiple fixed drug eruption. Because of the intractable nature of his chronic rhinitis without treatment, dupilumab was continued. His erythematous macules were re-evoked several hours after repeated dupilumab administration, indicating a positive result in the challenge test. Therefore, the diagnosis of multiple fixed drug eruption was made. Due to the efficacy against his chronic rhinitis, dupilumab was continued with topical steroid application for his skin eruption. Fortunately, his skin eruption gradually improved during continuous dupilumab administration without discontinuation of other oral medications (Fig. 1E, F).
DISCUSSION
Allergic cutaneous reactions due to dupilumab are recognized in a limited number of reported cases, with a total of 9 cases of allergic cutaneous reactions during dupilumab treatment (Table SI). Only our case showed fixed drug eruptions. The majority of skin types were lichenoid reactions (2 cases) and urticaria (2 cases), followed by purpuritic-type drug eruption (1 case), acute generalized exanthematous pustulosis (1 case), erythema multiforme (1 case), angioedema (1 case), and fixed drug eruption (1 case). Among them, 2 cases were able to continue dupilumab administration. As a possible mechanism, IL-4-deficiency impaired Th1-mediated delayed hypersensitivity reactions (5), indicating that IL-4 negative regulation by dupilumab might contribute to the impairment of fixed drug eruptions. Additionally, as patients with fixed drug eruptions are reported to be desensitized through repeated administration of the causative agents in previous human reports (6), our case also exhibited an improvement in re-evoked skin eruption with repeated dupilumab administration, suggesting a possible mechanism of desensitization against dupilumab. Therefore, a Th1-mediated delayed hypersensitivity reaction to dupilumab might allow continued treatment in cases of mild forms of drug eruption.
In sum, dupilumab is extensively employed for various human allergic diseases, and clinicians should be aware of the rare incidence of cutaneous allergic reactions during treatment. In such cases, the possibility of continuing dupilumab treatment may depend on the severity of the drug eruption.
REFERENCES
- Sawada Y, Nakamura M, Tokura Y. Generalized fixed drug eruption caused by pazufloxacin. Acta Derm Venereol 2011; 91: 600–601.
- Shiohara T. Fixed drug eruption: pathogenesis and diagnostic tests. Curr Opin Allergy Clin Immunol 2009; 9: 316–321.
- Bhadresha S, Hughes AJ, McMahon L, Reeken S, Natkunarajah N. Fixed drug eruption secondary to adalimumab. Clin Exp Dermatol 2021; 46: 366–368.
- Cork MJ, Lockshin B, Pinter A, Chen Z, Shumel B, Prescilla R. Clinically meaningful responses to dupilumab among children aged 6 months to 5 years with moderate-to-severe atopic dermatitis who did not achieve clear or almost clear skin according to the investigator’s global assessment: a post hoc analysis of a phase 3 trial. Acta Derm Venereol 2024; 104: adv13467.
- Dieli F, Sireci G, Scirè E, Salerno A, Bellavia A. Impaired contact hypersensitivity to trinitrochlorobenzene in interleukin-4-deficient mice. Immunology 1999; 98: 71–79.
- Yung CC, Watts TJ, Haque R. Successful desensitization to metronidazole in a patient with generalized fixed drug eruption. J Allergy Clin Immunol Pract 2020; 8: 769–771.e1.