Early detection of melanoma metastasis is essential in order to initiate treatment and improve patient prognosis. The aim of this study was to determine the diagnostic accuracy of different image-guided biopsy techniques in patients with melanoma. A cohort study of patients diagnosed with melanoma who had undergone image-guided biopsies (ultrasound-guided fine-needle aspiration cytology, ultrasound-guided core-needle biopsy, computerized tomography-guided fine-needle aspiration cytology and computerized tomography-guided core-needle biopsy) to detect melanoma metastasis between 2004 and 2021 was conducted. The reference standard was histological confirmation and/or clinical-radiological follow-up. Sensitivity, specificity, positive and negative predictive values were calculated. A total of 600 image-guided biopsies performed on 460 patients were included for analysis. Locoregional lesions represented 459 (76.5%) biopsies, and 141 (23.5%) were distant lesions. Of the included biopsies, 49 (8.2%) were insufficient for diagnosis. Overall, sensitivity and specificity were 92% (95% confidence interval 89–94) and 96% (95% confidence interval 91–99), respectively. Sensitivity sub-analyses revealed lower diagnostic accuracy values in the lung, inguinal lymph nodes, and computerized tomography-guided lesions under 1 cm. Limitations include spontaneous metastasis regression and arbitrary minimum follow-up period. Image-guided biopsies in patients with melanoma have high sensitivity and specificity for detection of regional or distant metastasis. Tissue type, location and tumour burden may influence the diagnostic accuracy of the test.
Key words: core-needle biopsy; diagnostic imaging; fine-needle aspiration biopsy; image-guided biopsy; melanoma; metastasis.
Accepted Nov 17, 2022; Published Dec 13, 2022
Acta Derm Venereol 2022; 102: adv00833.
DOI: 10.2340/actadv.v102.3981
Corr: Sebastian Podlipnik, Melanoma Unit, Dermatology Department, Hospital Clínic Barcelona, University of Barcelona, C/Villarroel 170, ES-08036 Barcelona, Spain. E-mail: podlipnik@clinic.cat
SIGNIFICANCE
Early detection of melanoma metastasis is essential to initiate treatment and improve patient prognosis. Prior studies of image-guided biopsies for detection of melanoma metastasis have been performed before the introduction of novel therapies, which include a limited variety of image-guided biopsy techniques and exclude inconclusive test results. The current study evaluated the diagnostic accuracy of a wide variety of image-guided biopsy techniques and included inconclusive test results in the statistical analysis. Image-guided biopsies have high sensitivity and specificity for the detection of melanoma metastases. Diagnostic accuracy may vary according to the tissue type, location, and tumour burden.
INTRODUCTION
Cutaneous melanoma (CM) is a potentially lethal skin cancer whose incidence rates have continued to increase steadily worldwide over recent years (1, 2). Development of locoregional or distant metastasis portends a detrimental impact on survival (3). Since the publication of the second Multicenter Selective Lymphadenectomy Trial (MSLT-II), complete lymphadenectomy in sentinel-positive patients has largely been abandoned (4). Hence, the importance of establishing intensive surveillance schedules for high-risk melanoma patients, shown to be useful for the early detection of metastatic disease (5). This may lead to earlier treatment with less tumoural burden, potentially leading to an improved overall survival rate in these patients (6).
Where possible, pathological confirmation of nodal or distant metastatic recurrence is advised to ensure correct management (7). Image-guided biopsies represent safe, cost-effective procedures, which have been shown to be highly sensitive and specific for the detection of melanoma metastasis to lymph nodes, and to a wide variety of organs (8). The diagnostic accuracy of these tests in confirming pathological staging has been reported in a limited number of studies (9), published prior to the MSLT-II trial and prior to the use of immunotherapy. Moreover, the vast majority of published studies have only studied ultrasound-guided fine-needle aspiration cytology (US-FNAC), and have excluded inconclusive biopsies from the statistical analysis (8, 10).
The aim of this study was to provide real-life characterization of the diagnostic accuracy of image-guided biopsies for initial staging and follow-up of patients with melanoma.
MATERIALS AND METHODS
Study design and participants
All patients seen at the dermatology unit of a referral university hospital in Barcelona, Spain, with a diagnosis of melanoma between November 2004 and February 2021 were retrospectively reviewed. Patients who underwent image-guided biopsies during initial staging or follow-up of lesions clinically and/or in whom radiology led to suspicion of melanoma metastasis, were included. Exclusion criteria were: biopsies obtained for research purposes, indicated for intralesional treatment, performed with the suspicion of metastasis from a neoplasm other than melanoma, or with less than a 4-month follow-up period after the procedure. This time-frame was chosen based on previous publications assessing metastatic disease after an invasive melanoma diagnosis, although slow progressive disease can occur and a longer-term follow-up is preferred (11).
Test methods
When a new clinically palpable and/or radiologically detectable lesion was found, raising suspicion of melanoma metastasis, the patient was referred to the radiology unit for histological confirmation via image-guided biopsy. The most appropriate imaging and biopsy technique was chosen by the interventional radiologist, based on the number of suspicious lesions, affected tissue type, location, tumour burden, and the need to carry out molecular studies.
The procedure was performed by a radiologist specialized in the anatomical area to be biopsied (Fig. S1). From fine-needle aspiration cytology (FNAC) material, at least 4 cytological smears were evaluated and both Diff-Quik and Papanicolaou stains were carried out. Core needle biopsies were formalin-fixed, paraffin-embedded, and processed following standard procedures in the pathology laboratory. Several sections were performed and stained with haematoxylin-eosin. Where necessary for diagnosis, and sufficient material available, specific immunohistochemical markers (S-100 and MART-1) were performed. Biopsy specimens were examined and diagnosed by expert pathologists in melanocytic lesions, as follows: (i) melanoma metastasis; (ii) compatible with melanoma metastasis; (iii) atypical cells suspicious of melanoma metastasis; (iv) absence of malignant cells; or (v) insufficient for diagnosis if the smears or core needle samples lacked sufficient material for diagnosis.
The diagnostic accuracy of image-guided biopsies was assessed by comparison with 2 reference standards: histopathological confirmation of the excised lesion (complete lymph node dissection or tumourectomy), and clinical and/or radiological follow-up when histopathological material was not available. The lesion was considered benign if it remained stable in size or resolved without treatment, and malignant if it increased in size and/or was associated with new metastatic lesions. The test was considered inconclusive when there was insufficient material for diagnosis.
Statistical analysis
Basal demographic characteristics, clinical and histopathological features of primary tumour and metastasis, biopsy sample method used, lesion size, tissue type and location, and gold standard confirmation were recorded.
Statistical tests of sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio and negative likelihood ratio were conducted. Definitions for false-negative, false-positive, true-positive, and true-negative results are specified in Table SI. Inconclusive test results were also included in the diagnostic accuracy analyses. Two different scenarios were contemplated: a best-case scenario in which “inconclusive diagnostics” were considered negative for melanoma, and a worst-case scenario in which these cases were considered positive for melanoma, in order to evaluate their influence on the diagnostic accuracy of the test. Diagnostic accuracy sub-analyses were conducted to assess the performance of image-guided biopsies under different conditions.
The Hospital Clinic of Barcelona Clinical Research Ethics Committee approved the study and research protocol. All procedures performed in studies involving human participants were in accordance with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. This study was performed following the 2015 Standards for Reporting of Diagnostic Accuracy Studies (STARD) guidelines (12).
RESULTS
Patient characteristics
Between December 2004 and February 2021, 777 image-guided biopsies from 540 patients with a history of histologically proven melanoma were screened for initial eligibility. A total of 177 biopsies were excluded due to performance for research purposes, suspicion of metastasis other than melanoma, intralesional treatment, insufficient follow-up time and insufficient information. A total of 600 biopsies performed on 460 patients, 190 (41.3%) women and 270 (58.7%) men, were finally included for analysis (Fig. 1). Mean time between melanoma diagnosis and image-guided biopsies was 40.7 months (standard deviation (SD) 48.9), with a total person-year follow-up time of 4.7 (median 3.7, interquartile range (IQR) 1.5–7.7 years). Median Breslow Index for primary melanomas was 2.8 mm (IQR 1.6–5.0), with superficial spreading and nodular histological subtypes being the most frequent (49.9% and 29.6%, respectively), followed by acral lentiginous (9.3%) and lentiginous malignant (3.2%). The most frequent stage according to the AJCC 2017 8th edition staging system was stage III (46%) (Table I).
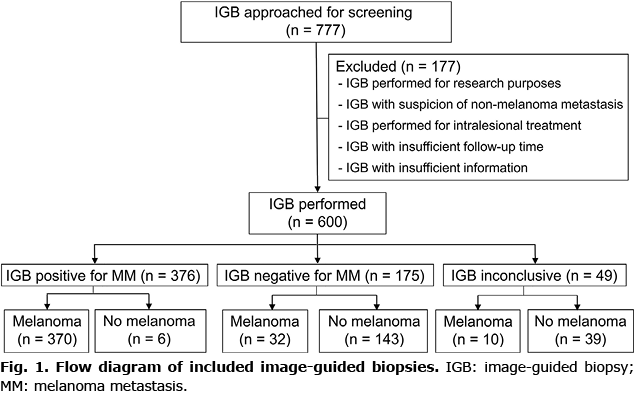
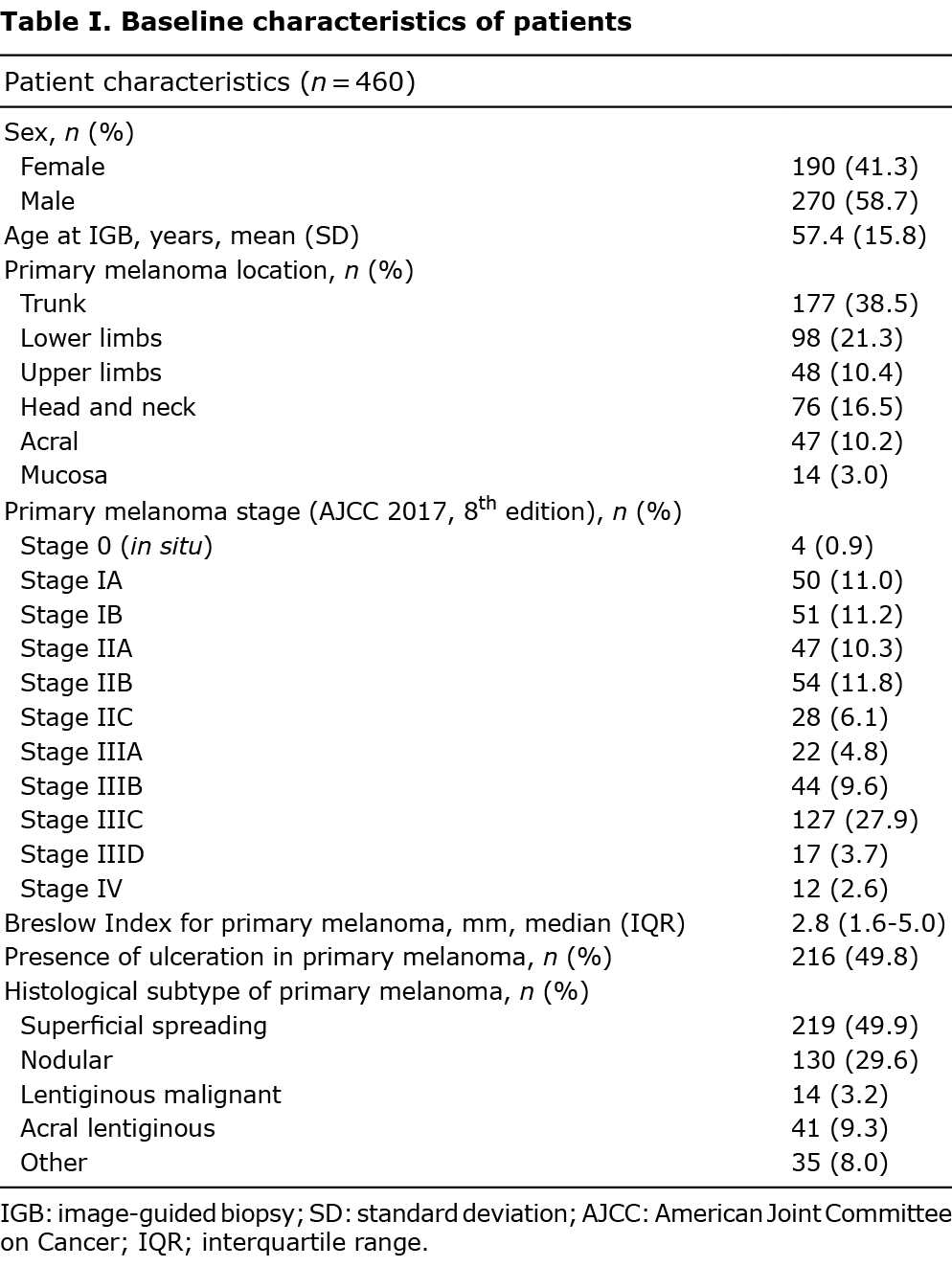
Image-guided biopsies
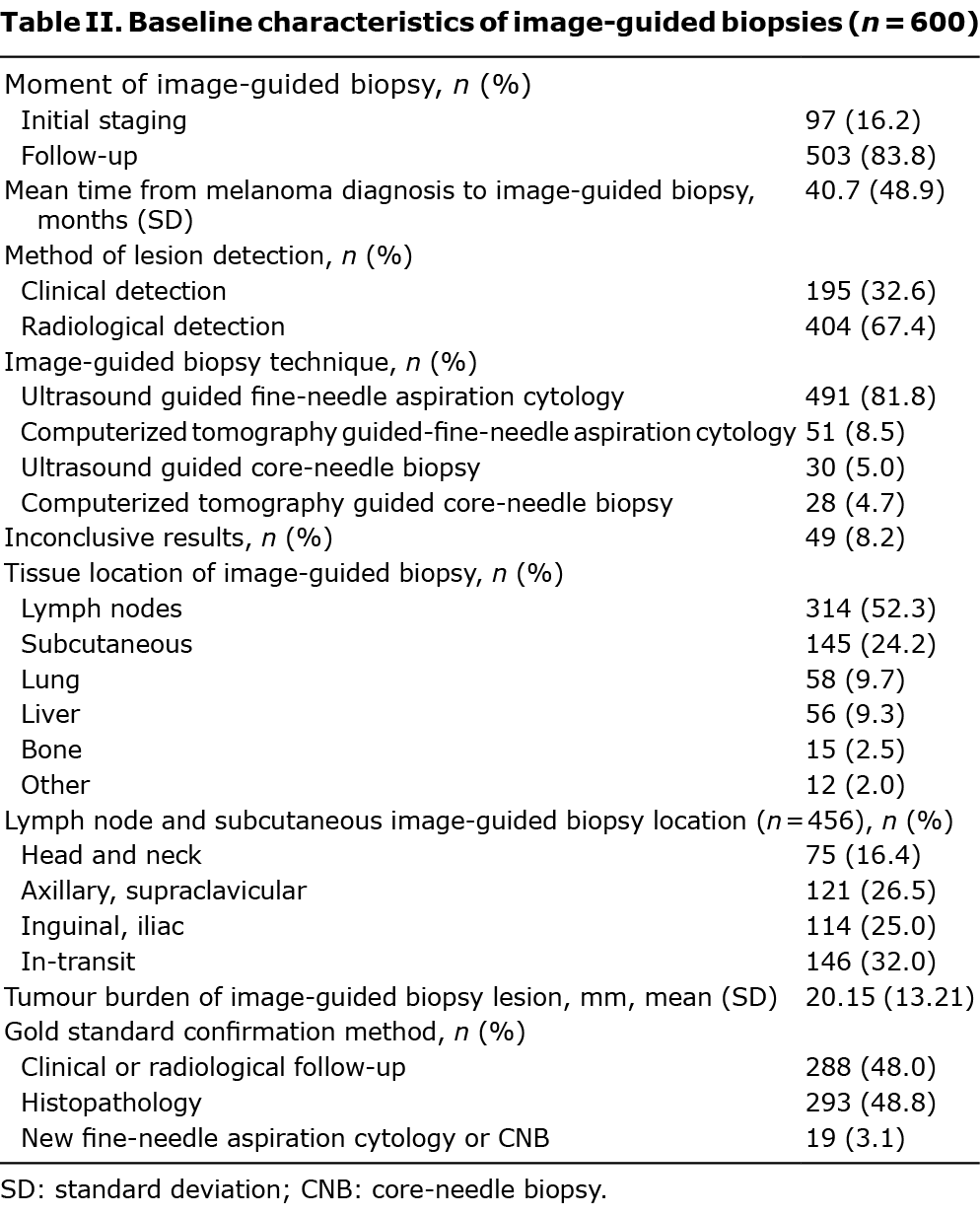
Among the 600 image-guided biopsies performed, 83.8% were carried out during follow-up. Biopsies were performed based on radiological detection (67.4%) and clinical examination (32.6%). The preferred technique was US-FNAC (491, 81.8%), followed by computerized tomography-guided fine-needle aspiration cytology (CT-FNAC) (51, 8.5%), and ultrasound-guided and computerized tomography (CT)-guided core-needle biopsy (US-CNB and CT-CNB) (5% and 4.7%, respectively). Image-guided biopsies were mostly performed on lymph nodes (314, 52.3%), followed by subcutaneous nodules (145, 24.2%), lung (58, 9.7%) and liver (56, 9.3%) lesions. Of the included biopsies, 49 (8.2%) were insufficient for diagnosis. Image-guided biopsies were confirmed by histopathology in 48.8% cases, clinical follow-up in 48%, and with a new biopsy in 19 cases (Table II).
Diagnostic accuracy of image-guided biopsies in patients with melanoma
A total of 376 biopsies were compatible with melanoma, with only 6 (1%) being false-positive results. Among the 175 biopsies negative for melanoma, 32 (5.3% of all image-guided biopsies performed) were false-negative (Fig. 2). Overall sensitivity for the 551 image-guided biopsies excluding inconclusive results was 92% (95% CI 89–94%), and specificity was 96% (95% CI 91–99%). PPV was 98% (95% CI 97–99%) and NPV was 82% (95% CI 75–87%) (Table III).
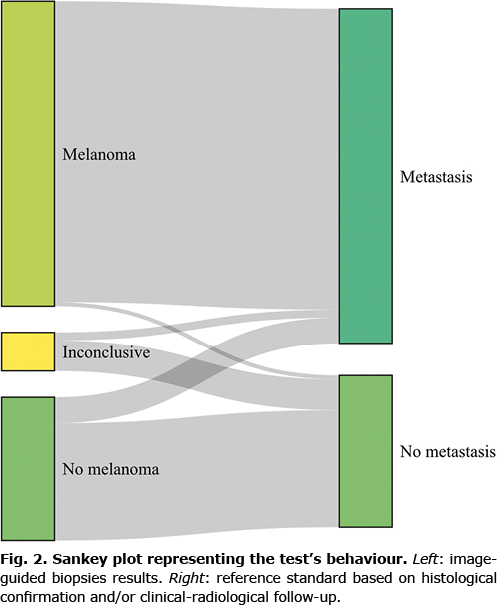
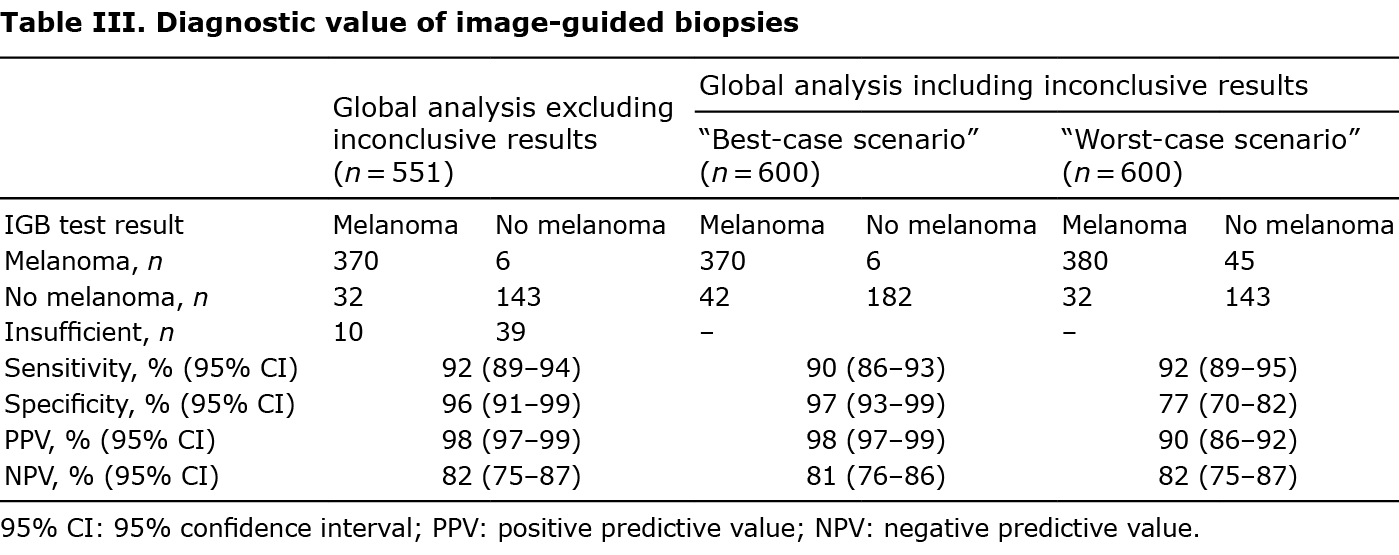
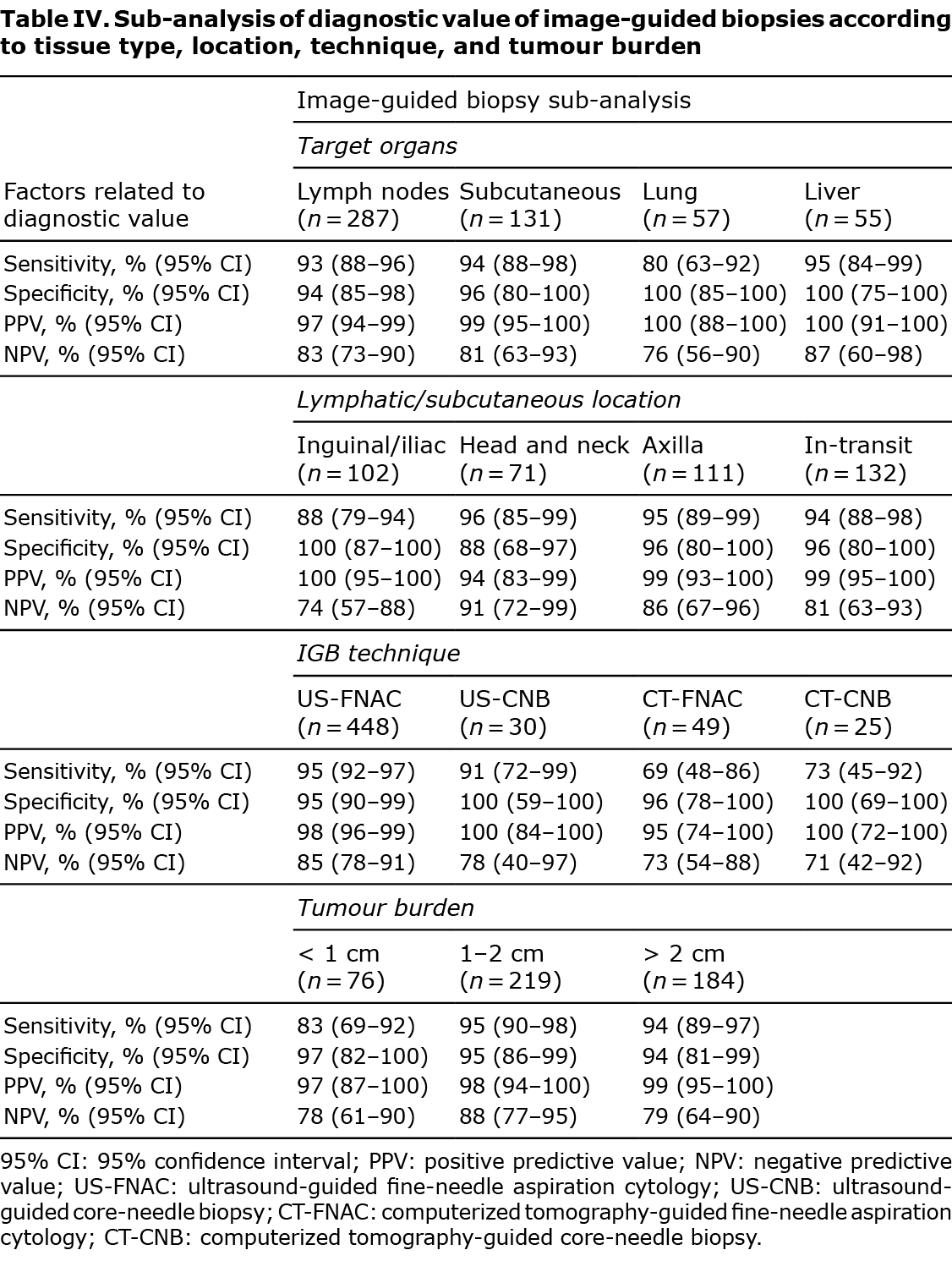
Sensitivity sub-analyses were performed regarding tissue type, location, size of lesion biopsied and by the image-guided biopsy technique implemented (Table IV). High values of sensitivity, specificity, PPV and NPV were consistent among the different tissue types biopsied, while results from the lung showed lower sensitivity (80%) and NPV (76%). Image-guided biopsies of inguinal lymph nodes had a lower sensitivity (88%) and NPV (74%), but 100% specificity and PPV, indicating that a result positive for melanoma in this region can be considered certain, whereas a negative result should be treated with caution. Among image-guided biopsy techniques implemented, US-guided biopsies presented high values of sensitivity, specificity, PPV and NPV. CT-guided biopsies gave high values of specificity (96% and 100% for CT-FNAC and CT-CNB, respectively) and PPV (95% and 100% for CT-FNAC and CT-CNB, respectively), but lower values of sensitivity (69% and 73% for CT-FNAC and CT-CNB, respectively) and NPV (73% and 71% for CT-FNAC and CT-CNB, respectively). Image-guided biopsies of lesions under 1 cm presented lower sensitivity (83%) and NPV (78%) (Table IV). Of the 49 image-guided biopsies catalogued as inconclusive, only 10 (20.4%) were finally diagnosed as melanoma, the other 39 (79.6%) were catalogued as benign after histological analysis or follow-up (Fig. 2). Verification was determined by clinical or radiological follow-up for 26 (53.1%) cases inconclusive results, excision in 19 (38.8%) cases and new image-guided biopsy in 5 (10.2%) cases. In the analysis including inconclusive results in the best-case scenario, diagnostic accuracy values were similar to those found in the global analysis without inconclusive results. However, in the worst-case scenario, considered the closest situation to real-life, specificity (77%) and PPV (90%) were significantly lower compared with the overall results (Table III).
Characteristics of false-positives are summarized in Table SII. Characteristics of false-negative results, repeated image-guided biopsies and inconclusive results are shown in Table SIII.
DISCUSSION
This study shows that image-guided biopsies are a safe and highly sensitive and specific method to detect melanoma metastasis in patients with melanoma, even when inconclusive cases are integrated into the analysis. Diagnostic accuracy may differ depending on biopsied tissue type, lymph node location, tumour burden and the image-guided biopsy technique. Another factor that may influence diagnostic accuracy, but has not been evaluated in the current study, is operator experience in image-guided biopsies (13, 14). Lower sensitivity and NPV was found for image-guided biopsies performed on the lung, inguinal or iliac lymph nodes, lesions smaller than 1 cm in maximum diameter and CT-guided biopsies.
Previous studies have demonstrated the high diagnostic accuracy of palpation-guided and US-guided FNAC and US-guided CNB of lymph nodes and other lesions suspicious of melanoma metastasis (15–17). Mean sensitivity and specificity values from previous studies are 97% and 99%, respectively, similar to the findings in the current study (9). These studies do not include CT-guided image-guided biopsies, and only 1 has evaluated the diagnostic accuracy of US-CNB in patients with melanoma (17). A varying number of inconclusive results (0–18.8%) have been reported in previous studies (8, 10, 18–20). However, only Voit et al. (21) have included the inconclusive results in their diagnostic accuracy analysis, considering them as test negative. Recommendations for the publication of diagnostic accuracy studies recommend their inclusion, since they are an indirect indicator of the feasibility of a test, and diagnostic accuracy bias can occur if they are ignored (13).
One of the strengths of the current study is that it includes a wide variety of image-guided biopsy techniques, biopsied tissue types, and patients with all stages of melanoma. The current study also included inconclusive results in the diagnostic accuracy analysis (8, 10, 18–21). It was decided to include these results in the diagnostic accuracy analysis, contemplating a best- and worst-case scenario. In the former, inconclusive results were considered negative for melanoma; therefore further examinations would not be requested. In the latter, inconclusive test results were considered positive for melanoma, and thus complete excision or further radiological investigations would be indicated. A lower specificity and PPV was observed in the worst-case scenario, reflecting that most inconclusive results are finally negative for melanoma, but given an inconclusive result, a new biopsy or excision would be recommended, taking into account the patient’s clinical situation and the degree of clinical or radiological suspicion.
High diagnostic accuracy values were found for image-guided biopsies in patients with melanoma: 92% (95% CI 89–94) and 96% (95% CI 91–99) of sensitivity and specificity, respectively. Factors contributing to improved sensitivity values are superficial anatomical location, availability of immunohistochemical studies, and the experience of the reporting pathologist (8). Diagnostic accuracy is largely dependent on operator skill (13, 14). Studies assessing the utility of image-guided biopsies for the diagnosis of metastatic melanoma have reported whether the operator was a radiologist or cytologist (8, 10, 22), but none have compared operator experience and diagnostic accuracy in this subset of patients. However, cancer detection rates between trained physicians and inexperienced physicians performing FNAC on breast or thyroid lesions have been shown to be significantly lower for inexperienced physicians (13, 14). Radiologists performing image-guided biopsies at our centre have extensive experience, and pathologists are highly trained in cytological studies. This could explain the high rates of sensitivity and specificity in the current cohort. Lower sensitivity and NPV was obtained for lung biopsies, probably due to inadequate sampling and low cellularity obtained from these lesions (20). Even though the current study did not determine whether lung lesions were generally smaller and/or more deeply located, in our experience, this could explain the lower sensitivity values. CT-guided biopsies were also found to produce lower sensitivity values. Most CT-guided biopsies correspond to deeply located lesions, which implies greater technical difficulty and could explain the current results. Inguinal lymph nodes had lower sensitivity and NPV. This is inconsistent with reports in the literature, where the axillary region has been signalled as a frequent site of false-negative results (9, 23). Tumour burden affected the sensitivity of imaging-guided biopsies, with 83% (95% CI 69–92) sensitivity in metastases smaller than 10 mm, compared with 95% (95% CI 90–98) in larger lesions. Similarly, Voit et al. (21) found 99% sensitivity for suspicious melanoma metastasis lesions > 0 mm vs 94.6% for lesions < 10 mm in diameter.
False-positive rates of 0% to 2% have been reported for FNAC of melanoma metastases (15). A false-positive rate of 1%, corresponding to 5 non-malignant lesions and 1 second malignancy was found. Previous studies have claimed that the most common cause of false-positive result is a second malignancy (8, 18). Other authors offer different explanations, including paucicellular smears, overinterpretation of reactive fibroblasts and benign spindle cell lesions, and the presence of atypical histiocytes, mimicking melanoma cells (8, 9, 18). A difficulty exists in establishing an accurate diagnosis in false-positive results. Melanoma metastases regression is an infrequent, but possible, event, which can be due to operative trauma, infection, and the host’s immunological response (24). In rare cases, we have observed metastasis regression in the period of time between the imaging-guided biopsy and histopathological confirmation. In the current study, only 2 of the 6 false-positive test results presented inflammatory changes on histological confirmation.
The false-negative rate in the current study (5.3%) is within the reported range in the literature (0–12%) (15). Inadequate sampling is the reason for the vast majority of false-negative cases described (8, 18, 20). We hypothesize that image-guided biopsies were unable to show malignant tissue, due to anatomical difficulties, small and mobile nodes, the presence of metastases as only a proportion of the tumour burden, poor cellular yield, and the presence of scarring and necrotic tissue.
Limitations
Limitations of this study include an arbitrary minimum follow-up time after the image-guided biopsy of 4 months, after which a subset of false-negative results might arise due to slow progression of melanoma metastasis. The different paths taken by metastasis development and spontaneous metastasis regression make it difficult to establish the most useful minimum follow-up time to deliberate a false-positive or false-negative finding. Performing a multivariate analysis including lesion size, lesion location and imaging-biopsy technique would enable better interpretation of the current results. Operator expertise is a variable that might influence the diagnostic accuracy of image-guided biopsies, but this was not the aim of the current study.
Conclusion
This study demonstrates high sensitivity and specificity for image-guided biopsies of lesions suspicious of locoregional or distant metastasis in patients with melanoma. Image-guided biopsies represent minimally invasive, safe, cost-effective procedures, which allow the early detection and treatment of patients with metastatic disease. Tissue type, location and tumour burden may influence precision and must be considered when interpreting results.
ACKNOWLEDGEMENTS
The research at the Melanoma Unit from Hospital Clínic of Barcelona is partially funded by grants PI18/00419 and PI18/01077 from Fondo de Investigaciones Sanitarias (Spain), by the CIBER de Enfermedades Raras of the Instituto de Salud Carlos III, Spain, co-funded by ISCIII-Subdireccion General de Evaluacion and European Regional Development Fund (ERDF), “a way to make Europe”; by the Agency for Management of University and Research Grants (AGAUR) 2017_SGR_1134 of the Catalan Government, Spain; by a grant from “Fundació La Marató de TV3, 201331-30” Catalonia, Spain; by the European Commission under the 6th Framework Programme, Contract No. LSHC-CT-2006-018702 (GenoMEL); by Centres de Recerca de Catalunya (CERCA) Programme/Generalitat de Catalunya; by a research grant from “Fundación Científica de la Asociación Española Contra el Cáncer” GCB15152978SOEN, Spain.
IRB approval status. HCB/2015/0298.
The authors have no conflicts of interest to declare.
REFERENCES
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020; 70: 7–30.
- Whiteman DC, Green AC, Olsen CM. The growing burden of invasive melanoma: projections of incidence rates and numbers of new cases in six susceptible populations through 2031. J Invest Dermatol 2016; 136: 1161–1171.
- Melanoma of the Skin–Cancer Stat Facts. SEER [cited 2022 Jan 3] Available from: https://seer.cancer.gov/statfacts/html/melan.html.
- Faries MB, Thompson JF, Cochran AJ, Andtbacka RH, Mozzillo N, Zager JS, et al. Completion dissection or observation for sentinel-node metastasis in melanoma. N Engl J Med 2017; 376: 2211–2222.
- Podlipnik S, Carrera C, Sánchez M, Arguis P, Olondo ML, Vilana R, et al. Performance of diagnostic tests in an intensive follow-up protocol for patients with American Joint Committee on Cancer (AJCC) stage IIB, IIC, and III localized primary melanoma: a prospective cohort study. J Am Acad Dermatol 2016; 75: 516–524.
- Ascierto PA, Long GV, Robert C, Brady B, Dutriaux C, Di Giacomo AM, et al. Survival outcomes in patients with previously untreated BRAF wild-type advanced melanoma treated with nivolumab therapy: three-year follow-up of a randomized phase 3 trial. JAMA Oncol 2019; 5: 187–194.
- National Comprehensive Cancer Network (NCCN). Melanoma: cutaneous. Version 1.2022. [cited 2022 Jan 3]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf.
- Murali R, Doubrovsky A, Watson GF, McKenzie PR, Lee CS, McLeod DJ, et al. Diagnosis of metastatic melanoma by fine-needle biopsy: analysis of 2,204 cases. Am J Clin Pathol 2007; 127: 385–397.
- Hall BJ, Schmidt RL, Sharma RR, Layfield LJ. Fine-needle aspiration cytology for the diagnosis of metastatic melanoma. Am J Clin Pathol 2013; 140: 635–642.
- Dalle S, Paulin C, Lapras V, Balme B, Ronger-Savle S, Thomas L. Fine-needle aspiration biopsy with ultrasound guidance in patients with malignant melanoma and palpable lymph nodes. Br J Dermatol 2006; 155: 552–556.
- Riquelme-Mc Loughlin C, Podlipnik S, Bosch-Amate X, Riera-Monroig J, Barreiro A, Espinosa N, et al. Diagnostic accuracy of imaging studies for initial staging of T2b to T4b melanoma patients: a cross-sectional study. J Am Acad Dermatol 2019; 81: 1330–1338.
- Cohen JF, Korevaar DA, Altman DG, Bruns DE, Gatsonis CA, Hooft L, et al. STARD 2015 guidelines for reporting diagnostic accuracy studies: explanation and elaboration. BMJ Open 2016; 6: e012799.
- De Fiori E, Rampinelli C, Turco F, Bonello L, Bellomi M. Role of operator experience in ultrasound-guided fine-needle aspiration biopsy of the thyroid. Radiol Med (Torino) 2010; 115: 612–618.
- Ljung BM, Drejet A, Chiampi N, Jeffrey J, Goodson WH, Chew K, et al. Diagnostic accuracy of fine-needle aspiration biopsy is determined by physician training in sampling technique. Cancer 2001; 93: 263–268.
- Murali R, Thompson JF, Uren RF, Scolyer RA. Fine-needle biopsy of metastatic melanoma: clinical use and new applications. Lancet Oncol 2010; 11: 391–400.
- Voit CA, van Akkooi ACJ, Eggermont AMM, Schäfer-Hesterberg G, Kron M, Ulrich J, et al. Fine needle aspiration cytology of palpable and nonpalpable lymph nodes to detect metastatic melanoma. J Natl Cancer Inst 2011; 103: 1771–1777.
- Bohelay G, Battistella M, Pagès C, de Margerie-Mellon C, Basset-Seguin N, Viguier M, et al. Ultrasound-guided core needle biopsy of superficial lymph nodes: an alternative to fine-needle aspiration cytology for the diagnosis of lymph node metastasis in cutaneous melanoma. Melanoma Res 2015; 25: 519–527.
- Rodrigues LK, Leong SP, Ljung BM, Sagebiel RW, Burnside N, Hu T, et al. Fine needle aspiration in the diagnosis of metastatic melanoma. J Am Acad Dermatol 2000; 42: 735–740.
- Basler GC, Fader DJ, Yahanda A, Sondak VK, Johnson TM. The utility of fine needle aspiration in the diagnosis of melanoma metastatic to lymph nodes. J Am Acad Dermatol 1997; 36: 403–408.
- Perry MD, Seigler HF, Johnston WW. Diagnosis of metastatic malignant melanoma by fine needle aspiration biopsy: a clinical and pathologic correlation of 298 cases. J Natl Cancer Inst 1986; 77: 1013–1021.
- Voit C, Mayer T, Proebstle TM, Weber L, Kron M, Krupienski M, et al. Ultrasound-guided fine-needle aspiration cytology in the early detection of melanoma metastases. Cancer Cytopathol 2000; 90: 186–193.
- Cangiarella J, Symmans WF, Shapiro RL, Roses DF, Cohen JM, Chhieng D, et al. Aspiration biopsy and the clinical management of patients with malignant melanoma and palpable regional lymph nodes. Cancer 2000; 90: 162–166.
- Barkan GA, Rubin MA, Michael CW. Diagnosis of melanoma aspirates on ThinPrep: the University of Michigan experience. Diagn Cytopathol 2002; 26: 334–339.
- Kalialis LV, Drzewiecki KT, Klyver H. Spontaneous regression of metastases from melanoma: review of the literature. Melanoma Res 2009; 19: 275–282.