SHORT COMMUNICATION
Real-world Experience of Abrocitinib on Difficult-to-treat Hand Eczema in Chinese Patients
Yiting LI1#, Xi TAN1#, Shu NIE1, Xin TIAN2,3*, and Zhouwei WU1*
1Department of Dermatology, Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine, 100 Haining Rd, Shanghai 200080, China, 2Institute of Dermatology, Guangzhou Medical University, Guangzhou, China, and 3Department of Dermatology, Guangzhou Institute of Dermatology, 56 Hengfu Rd, Guangzhou 510095, China. *E-mail: xinzisue@163.com; zhouwei.wu@shgh.cn
#These authors contributed equally and should be considered as first authors.
Citation: Acta Derm Venereol 2024; 104: adv39822. DOI https://doi.org/10.2340/actadv.v104.39822.
Copyright: 2024 © The Author(s). Published by MJS Publishing, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/).
Submitted: Jan 14, 2024; Accepted: May 15, 2024; Published: Jun 8, 2024
Competing interests and funding: The authors have no conflicts of interest to declare.
INTRODUCTION
Chronic hand eczema (HE) is a distressing and pervasive dermatologic condition affecting approximately 10% of the global population, and effective treatment is challeng-ing (1). Severe itching, pain, and terrible appearance often result in decreased quality of life and a heavy psychosocial burden for patients (2,3). Conventional topical therapies for HE include emollients, topical corticosteroids, topical calcipotriol, and topical calcineurin inhibitors. Oral corticosteroids, alitretinoin, and immunosuppressants have been tried in difficult-to-treat patients, but the outcome is usually unsatisfactory and their long-term use was limited because of side effects (4). Recent literature showed that Janus kinase (JAK) inhibitors may be a potential treatment option for HE. Relevant clinical trials and case reports have shown the favourable efficacy and safety of delgocitinib, gusacitinib, baricitinib, and upadacitinib in treating HE (5–8). Abrocitinib is an oral selective JAK1 inhibitor recently approved for moderate-to-severe atopic dermatitis. It blocks IL-4, IL-13, IL-31, and IFN-γ pathways, which are involved in the pathogenesis of HE (9). However, the efficacy of abrocitinib on difficult-to-treat HE in real-world is limited (10, 11). Here, we reported the effectiveness and safety of abrocitinib on difficult-to-treat HE patients in daily practice.
MATERIALS AND METHODS
In this prospective, observational, single-centre cohort study, 12 adult patients with moderate-to-severe HE were enrolled from the Department of Dermatology of Shanghai General Hospital between March 2023 to May 2023. All patients met the diagnostic criteria for HE (12). According to medical history and patch test results, hand allergic and irritant contact dermatitis, hand psoriasis, and active hand infection were excluded. All these patients have failed at least 1 systemic treatment including corticosteroids, methotrexate, cyclosporine, and dupilumab. The study was conducted in accordance with the principles of the Declaration of Helsinki, and was approved by the Human Ethics Committee of Shanghai General Hospital (2023SQ293). Written informed consent was obtained from all patients.
All patients were administered abrocitinib 100 mg once daily for 16 weeks. All the patients had discontinued any systemic therapies for at least 3 months, and topical medications were also discontinued when abrocitinib treatment started. Patients were visited at the baseline, and weeks 2, 4, 8, 12, and 16.
The hand eczema severity index (HECSI) (13) and the 5-point Investigator’s Global Assessment (IGA) Scale (14) were used to assess the severity of HE by 2 independent dermatologists. Worst Itch Numeric Rating Scale (WI-NRS, 0 [no itch] to 10 [worst itch imaginable]) (15) was reported by the patient. The HECSI, WI-NRS, and IGA score were assessed at baseline, and weeks 2, 4, 8, 12, and 16. HECSI score improvement of ≥ 75% or ≥ 90% (HECSI-75, HECSI-90) was compared with baseline. Absolute cut-off scores were IGA ≤ 1 (clear or almost clear) and WI-NRS pruritus ≤ 4. Adverse events (AEs) were evaluated at each visit. Laboratory assessments including blood count, liver enzymes, and serum creatinine were performed at the baseline, and weeks 4 and 16.
IBM SPSS Statistics 26 (IBM Corp, Armonk, NY, USA) was used for statistical analysis. Changes in HECSI and WI-NRS scores from baseline to week 16 were compared with the Wilcoxon signed-rank test. A value of p < 0.05 was considered statistically significant. Figures were charted by GraphPad Prism 9 software (https://www.graphstats.net/graphpad-prism).
RESULTS
Patients and baseline characteristics. A total of 12 chronic HE patients (7 men and 5 women) were included. The mean age was 46.3 ± 14.1 years and the mean disease duration was 11.2 ± 7.3 years. According to the proposed classification of hand eczema, 9 patients had chronic recurrent vesicular hand eczema, and 3 patients had atopic hand eczema. Eight patients had comorbidities, the most common of which was allergic rhinitis (n = 6). All patients failed previous systemic treatment because of lack of response, intolerance, toxicity, or both lack of response and intolerance. Baseline characteristics and demographics are summarized in Table I.
Effectiveness. Six patients reached HECSI-75 at week 4 and all patients reached HECSI-90 after 16 weeks of treatment. The median HECSI score declined significantly from 136.0 (90.3–172.5) at baseline to 3.0 (1.0–7.3, p < 0.01) at week 16. Meanwhile, the median WI-NRS score changed significantly from 8.5 (7.3–10) to 0 (0–1.0, p < 0.01). The WI-NRS scores of all patients were ≤ 2 and 8 patients achieved no itch (0) at week 16. In addition, 9 patients achieved an IGA score of 0/1 and improved by at least 2 scales at week 16. Changes in HECSI, WI-NRS, and IGA score from baseline to week 16 are shown in Fig. 1 a–c). Fig. 1d shows the representative clinical photographs of 3 patients.
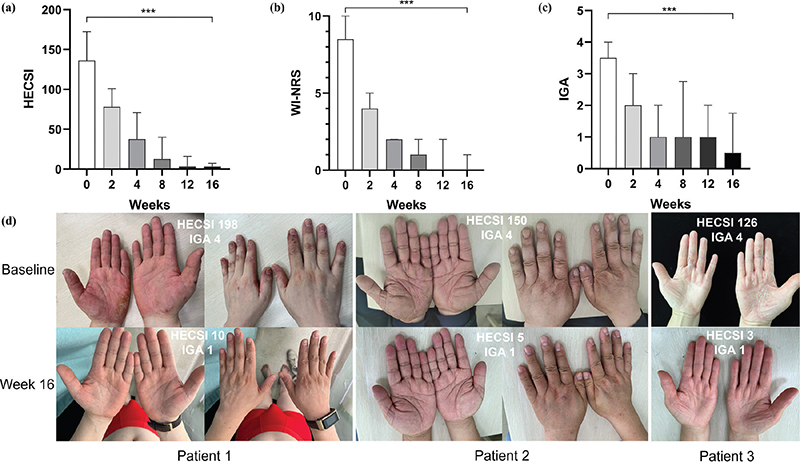
Fig. 1. (a) Median Hand Eczema Severity Index (HECSI) score over time. (b) Median Worst Itch Numeric Rating Scale (WI-NRS) score over time. (c) Median Investigator’s Global Assessment (IGA) score over time. (d) Representative clinical photographs of 3 patients at baseline and Week 16.
Safety. During the 16-week treatment, 7/12 of the patients experienced AEs, with 3 patients reporting more than 1 AE. The most frequently reported AEs was nausea (n = 4). Other AEs included headache (n = 3), acne (n = 1), dizziness (n = 1), and blurred vision (n = 1). No abnormal laboratory results were observed. No serious AEs were reported and no patients discontinued abrocitinib treatment.
DISCUSSION
In the current study, we evaluated the efficacy and safety of abrocitinib on 9 recurrent vesicular and 3 atopic HE patients who had failed previous systemic therapies. The response was significant in all aspects assessed, suggesting abrocitinib could improve both atopic and recurrent vesicular HE.
The study of abrocitinib on HE was limited. Sitaru et al. (10) reported a case of refractory hand and foot eczema was successfully treated with abrocitinib. Kamphuis et al. (11) demonstrated that abrocitinib improved HE in patients with AD in which 13/17 of the patients achieved clear or almost clear and 60% of the patients achieved HECSI 90 at week 16. In our study, all patients reached HECSI 90 and 9/12 of the patients achieved an IGA score of 0/1 at week 16. In addition, most patients in their study were given abrocitinib 200 mg once a day, while all our patients were administrated 100 mg once daily. The effectiveness of abrocitinib seemed better in our patients although the evaluation might be not accurate because of the smaller sample size of our study. However, we supposed that the discrepancy might be because the weight of patients was usually less in Chinese patients than that of European people, and the 9/12 patients in our study had recurrent vesicular hand eczema while the subtypes of patients in their study were mixed, for example contact subtypes were included.
There were some limitations in this study. First, the sample size of this study was small. Second, only short-term outcome was evaluated; the long-term maintenance effect of abrocitinib on HE needs further investigation.
In conclusion, abrocitinib, and other JAK inhibitors, seems not only to treat atopic HE, but also has a remarkable therapeutic effect on recurrent vesicular HE, and even on refractory HE that fails other systemic treatments.
ACKNOWLEDGEMENTS
This study was supported by Innovative Research Team of High-level Local Universities in Shanghai (SHSMU-ZDCX20211002) and Science and Technology Program of Guangzhou (Grant No. 2023A03J0468).
REFERENCES
- Agner T, Elsner P. Hand eczema: epidemiology, prognosis and prevention. J Eur Acad Dermatol Venereol 2020; 34: 4–12.
- Marron SE, Tomas-Aragones L, Navarro-Lopez J, Gieler U, Kupfer J, Dalgard FJ, et al. The psychosocial burden of hand eczema: data from a European dermatological multicentre study. Contact Dermatitis 2018; 78: 406–412.
- Quaade AS, Alinaghi F, Dietz JB, Erichsen CY, Johansen JD. Chronic hand eczema: a prevalent disease in the general population associated with reduced quality of life and poor overall health measures. Contact Dermatitis 2023; 89: 453–463.
- Cheng J, Facheris P, Ungar B, Guttman-Yassky E. Current emerging and investigational drugs for the treatment of chronic hand eczema. Expert Opin Investig Drugs 2022; 31: 843–853.
- Worm M, Thyssen JP, Schliemann S, Bauer A, Shi VY, Ehst B, et al. The pan-JAK inhibitor delgocitinib in a cream formulation demonstrates dose response in chronic hand eczema in a 16-week randomized phase IIb trial. Br J Dermatol 2022; 187: 42–51.
- Jimenez PA, Sofen HL, Bissonnette R, Lee M, Fowler J, Zammit DJ, et al. Oral spleen tyrosine kinase/Janus kinase inhibitor gusacitinib for the treatment of chronic hand eczema: results of a randomized phase 2 study. J Am Acad Dermatol 2023; 89: 235–242.
- Rosenberg FM, Loman L, Schuttelaar MLA. Baricitinib treatment of severe chronic hand eczema: two case reports. Contact Dermatitis 2022; 86: 419–421.
- Zalewski A, Szepietowski JC. Topical and systemic JAK inhibitors in hand eczema: a narrative review. Expert Rev Clin Immunol 2023; 19: 365–373.
- Vazquez ML, Kaila N, Strohbach JW, Trzupek JD, Brown MF, Flanagan ME, et al. Identification of N-{cis-3-[Methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino]cyclobutyl}propane-1-sulfonamide (PF-04965842): a selective JAK1 clinical candidate for the treatment of autoimmune diseases. J Med Chem 2018; 61: 1130–1152.
- Sitaru S, Preis S, Eberlein B. Successful treatment of atopic hand and foot eczema with oral Janus kinase 1 inhibition. Dermatitis 2023; 34: 560.
- Kamphuis E, Boesjes CM, Loman L, Kamsteeg M, Haeck I, Van Lynden-van Nes AMT, et al. Real-world experience of abrocitinib treatment in patients with atopic dermatitis and hand eczema: up to 28-week results from the BioDay Registry. Acta Derm Venereol 2024; 104: adv19454.
- Thyssen JP, Schuttelaar MLA, Alfonso JH, Andersen KE, Angelova-Fischer I, Arents BWM, et al. Guidelines for diagnosis, prevention, and treatment of hand eczema. Contact Dermatitis 2022; 86: 357–378.
- Held E, Skoet R, Johansen JD, Agner T. The hand eczema severity index (HECSI): a scoring system for clinical assessment of hand eczema. A study of inter- and intraobserver reliability. Br J Dermatol 2005; 152: 302–307.
- Futamura M, Leshem YA, Thomas KS, Nankervis H, Williams HC, Simpson EL. A systematic review of Investigator Global Assessment (IGA) in atopic dermatitis (AD) trials: many options, no standards. J Am Acad Dermatol 2016; 74: 288–294.
- Naegeli AN, Flood E, Tucker J, Devlen J, Edson-Heredia E. The Worst Itch Numeric Rating Scale for patients with moderate to severe plaque psoriasis or psoriatic arthritis. Int J Dermatol 2015; 54: 715–722