Recent studies have advanced our understanding of the clinical, histological and imaging characteristics of congenital haemangiomas (CHs), and have reported possible complications and atypical behaviour. The aim of this study is to describe the clinical, histological and ultrasound features of a series of CHs and to analyse their association with complications and atypical behaviour, with a view to providing diagnostic and management recommendations. The medical records, histology results and ultrasound images of all patients with CH diagnosed in the Dermatology Department of Alicante University General Hospital between 2006 and 2021 were retrospectively reviewed. A total of 18 patients were included, of whom 4 (22.2%) had complications. The most severe was 1 case with heart failure. There was a significant association between large CH size (> 5 cm) and the occurrence of complications (p = 0.019). The study identified 3 different lobule patterns, but found no relationship with CH subtype or other findings. The associations of venous ectasia, venous lakes and arteriovenous microshunts with occurrence of complications was borderline significant (p = 0.055). Study limitations were the small sample and the retrospective analysis. To conclude, haematological and cardiological assessment is indicated in large CHs and should be considered in CHs with ultrasound findings of venous ectasia, venous lakes or arteriovenous microshunts, as these cases present a greater risk of complications.
Key words: atypical behaviour; complications; congenital haemangioma; histology; ultrasound.
Accepted Dec 16, 2022; Published Jan 10, 2023
Acta Derm Venereol 2023; 103: adv00849.
DOI: 10.2340/actadv.v103.3983
Corr: Gloria Juan Carpena, Department of Dermatology, Morales Meseguer University Hospital, Avda/ Marqués de los Vélez s/n, ES-30008 Murcia, Spain. E-mail: gloria5289@gmail.com
SIGNIFICANCE
This study of the clinical, histological and ultrasound characteristics of congenital haemangiomas found that large size, venous ectasia, venous lakes and microshunts are associated with the occurrence of complications. Haematological and cardiological assessments are therefore warranted in cases of congenital haemangioma with any of the above-mentioned features.
INTRODUCTION
Congenital haemangiomas (CHs) are rare and benign vascular tumours that, unlike infantile haemangiomas, are fully formed at birth. Three subtypes have been distinguished according to clinical course: rapidly involuting CH (RICH), non-involuting CH (NICH) and partially involuting CH (PICH) (1–4). It was formerly believed that certain histological findings characterized specific subtypes of CH, but the current understanding is that different subtypes have overlapping histological features (2, 5–7). Ultrasound is a useful diagnostic technique in cases of suspected CH, although it does not enable us to distinguish between the different subtypes, it can help us to rule out other vascular abnormalities (8). Recent studies have described possible complications of CH, such as ulceration, bleeding, high-output heart failure and haematological alterations (9, 10). Atypical clinical manifestations have also been reported, including pain, segmental presentation, increased vascular markings, and expansion (11, 12). However, despite advances in the understanding of these tumours, no clear recommendations have been made for diagnosing CH subtype or for diagnosis and management of complicated CH.
This study aimed to describe the clinical, histological and ultrasound features of a series of CHs, and to examine the associations of these features with complications and atypical behaviour, with a view to proposing diagnostic and management recommendations.
MATERIALS AND METHODS
All patients diagnosed with CH in Alicante University General Hospital, Alicante, Spain, between 2006 and 2021 were selected retrospectively.
Medical records and serial clinical photographs were reviewed to collect data on epidemiological variables and clinical features (presentation, course and complications). RICHs were defined as CHs that flattened until completely disappearing, leaving only an area of skin atrophy or anetoderma; PICHs were defined as CHs that involuted partially, leaving a smaller bluish or skin-coloured lesion with or without residual course telangiectasia; and NICHs were defined as CHs that did not shrink during follow-up.
A dermatopathologist examined the available histological samples to identify the morphological characteristics of the capillary lobules and vascular lumina, the type of extralobular vessels, and other features (presence of hobnail endothelial cells, eosinophilic granules, fibrosis, hemosiderin, thrombosis, calcifications and arteriovenous shunts).
A radiologist reviewed the available ultrasound images. B mode had been used to evaluate lesion echogenicity, vessel visibility and size, and the presence of calcifications and thrombi. The vessels were classified into 3 groups: visible vessels (tubular structures measuring <1.5 mm in diameter), venous ectasia (tubular structures measuring 1.5–5 mm in diameter) and venous lakes (dilated and irregularly shaped vessels measuring > 5 mm in diameter). From the colour and spectral Doppler images, the radiologist collected data on vessel density per cm2 (< 2, 2–5 or > 5), flow type (venous or arterial), resistive index (cm/s) and the presence of arteriovenous shunts (arterialization of venous flow). Vessel density was measured on the image with a higher number of vessels in each case. The study also recorded whether patients had undergone other imaging tests (magnetic resonance imaging (MRI) scans of CHs or echocardiograms). Both the dermatopathologist and the radiologist were blinded to patients’ clinical data.
This study was performed as a subanalysis of a larger project (CEI PI2018/008) about infantile haemangiomas approved by the local ethics committee of Alicante University General Hospital (Alicante, Spain).
Statistical analysis
A descriptive analysis and a bivariate analysis were performed using the χ2 test and Fisher’s exact test, respectively. p-values <0.05 were considered statistically significant. All statistical analyses were performed using SPSS version 25.0.
RESULTS
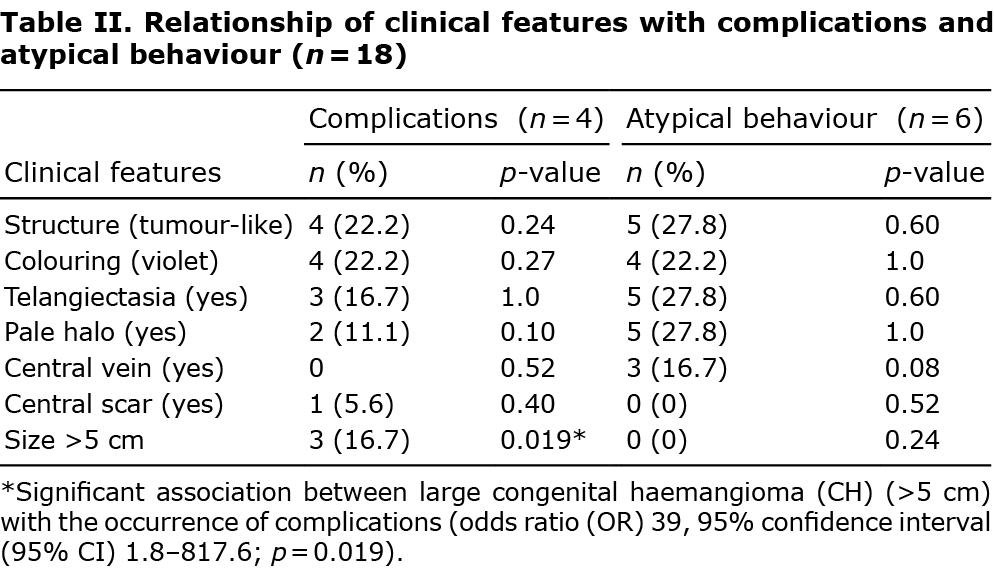
The current study series comprised 18 patients with CH: 5 RICHs (27.8%), 6 NICHs (33.3%) and 7 PICHs (38.9%). Five CHs (27.8%) were located on the trunk, 8 (44.4 %) on the upper limbs, 4 (22.2%) on the lower limbs and 1 (5.6%) on the head. Tumour-like lesions were more frequent than plaque-like lesions (66.7% vs 33.3), and violaceous colouring was more common than bluish colouring (72.2% vs 27.8%). Coarse telangiectasia was observed in 12 cases (66.7%), a pale halo in 15 (83.3%), a central vein in 4 (22.2%), and central scarring in 2 (11.1%). Four CHs (22.2%) were large (> 5 cm), and the mean ± standard deviation (SD) size was 3.75 ± 1.75 cm. The study did not observe a higher frequency of any clinical characteristics between the different subtypes (Table I).
The mean ± SD gestational age at delivery was 38.6 ± 1.73 weeks (range 34–41 weeks). Five patients were born by caesarean delivery (27.8%), in no case owing to the CH, while the remaining 13 (72.2%) were born by vaginal delivery. Mean ± SD weight at birth was 3,339 ± 586 g.
The follow-up period varied between 6 months and 8 years. Time to complete regression (in cases of RICH) or partial regression (PICH) ranged from 3 to 24 months, with a mean ± SD value of 12.36 ± 6.92 months. In patients with PICH, mean ± SD age at stabilization was 13.17 ± 6.05 months (range 6–24 months). In patients with RICH, mean ± SD age at complete regression was 11.40 ± 8.50 months (range 3–24 months).
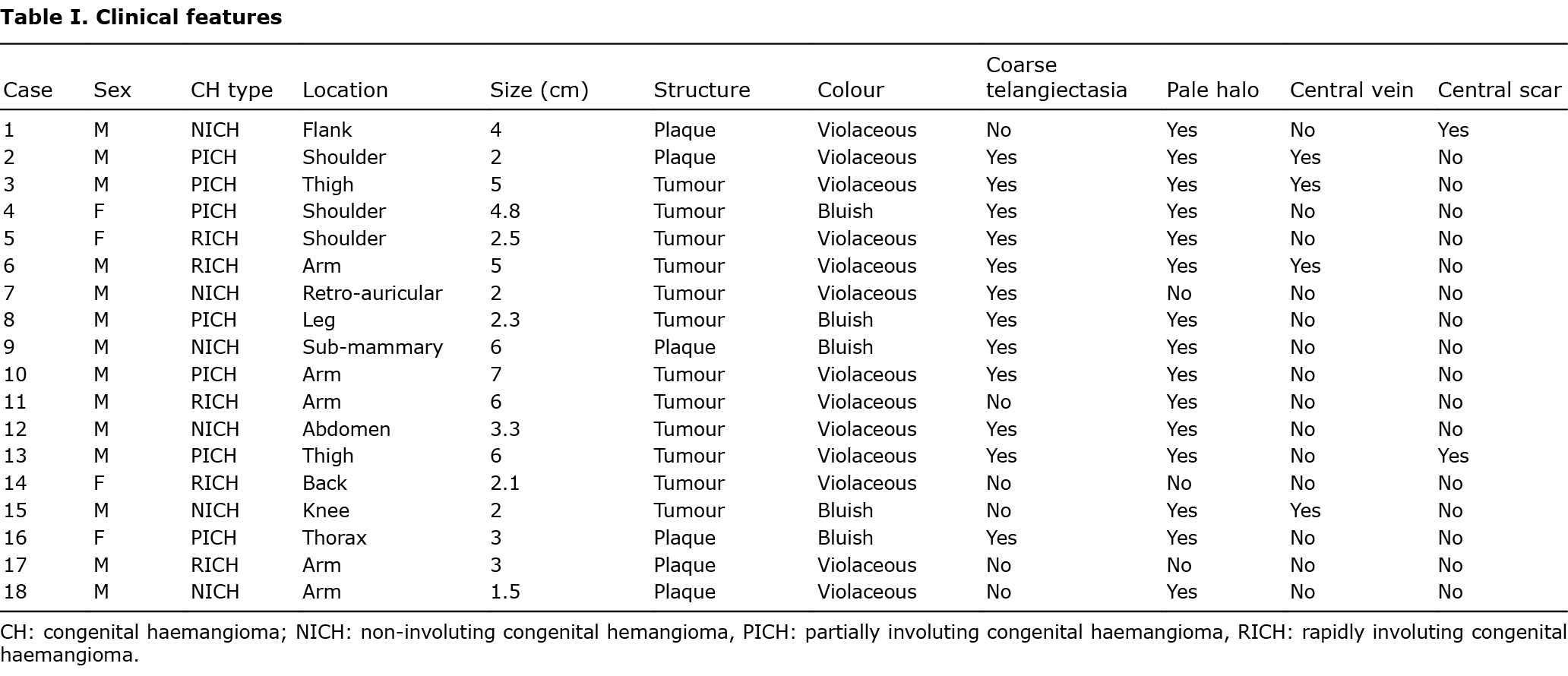
Four patients (22.2%) experienced complications, which included ulceration and bleeding (n = 2), bleeding without ulceration (n = 1), thrombocytopaenia (platelets=92,000) (n = 1) and high output heart failure (n = 1). The patient with heart failure (patient 13) also had ulceration and bleeding (Fig. 1). Large CH size (> 5 cm) was significantly associated with occurrence of complications (odds ratio (OR) 39, 95% CI 1.8–817.6; p = 0.019) (Table II).
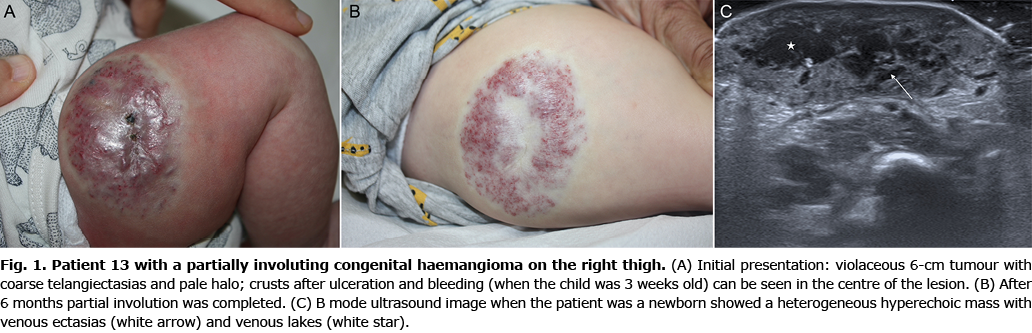
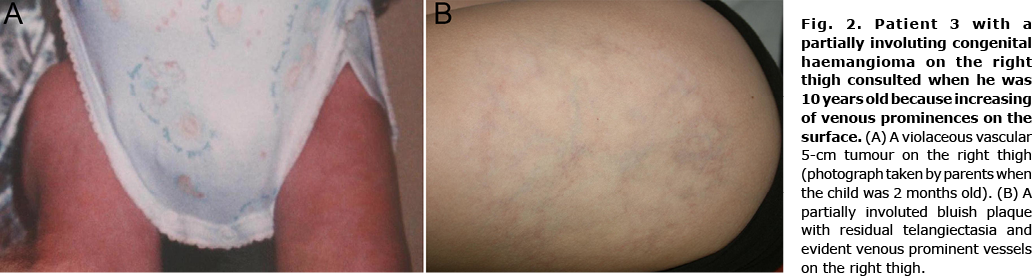
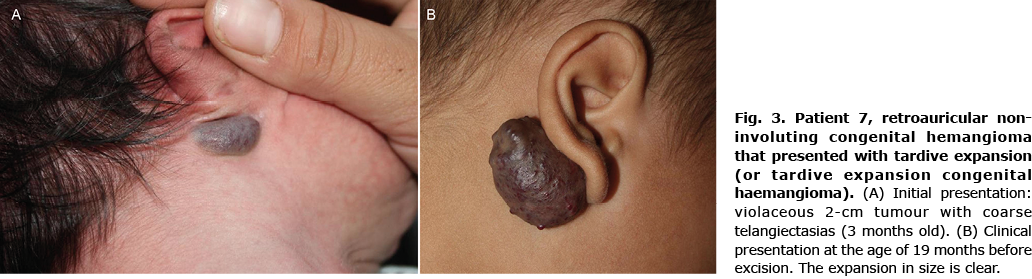
Six children (33.3%) showed signs of an atypical course: superficial draining veins becoming prominent (Fig. 2) (n = 2); telangiectasia appearing at 6 weeks of age (n = 1); pain (n = 3); and tardive expansion (n = 2) (Fig. 3). No relationship was found between any clinical characteristic and atypical course (Table II).
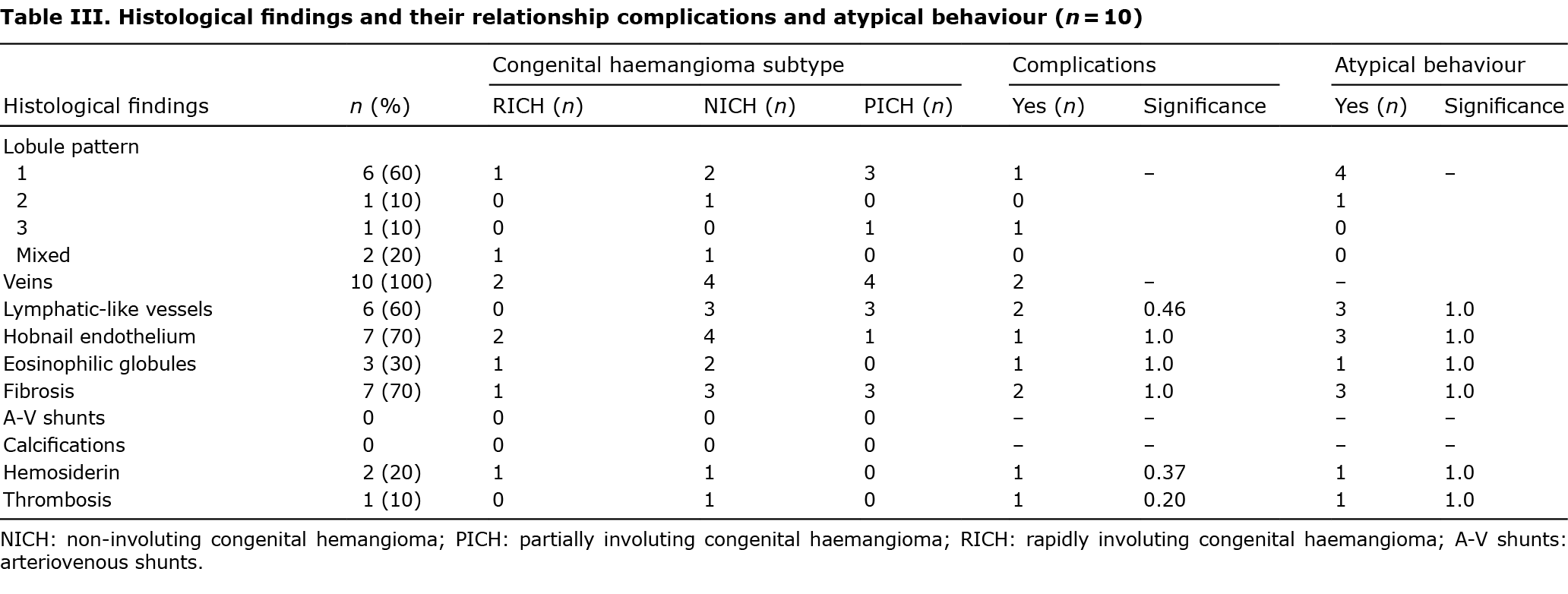
Pathology specimens had been taken from 10 patients (9 biopsies and 1 excision). All the CHs were composed of capillary lobules in the dermis (with possible extension to the hypodermis) and larger extralobular vessels. The histology findings are shown in Table III. Different patterns were identified based on lobule and lumen morphology: 60% of CHs had lobules composed of medium-sized capillaries with small and easily distinguishable lumina (pattern 1); pattern 2 consisted of lobules formed by a network of endothelial cells and pericytes, with a small number of medium-sized stellate lumina (n = 1); and pattern 3 comprised disseminated endothelial cells and pericytes with indistinguishable lumina (n = 1). Two CHs (20%) showed a mixed pattern (patterns 1 and 2) (Fig. 4). All cases had irregularly shaped extralobular vessels that appeared to be venous, with large, ectatic lumina; and 60% of lesions had apparently lymphatic-like vessels with winding lumina and thinner fibrous walls without smooth muscle. Perilobular or intralobular fibrosis and hobnail endothelial cells were each identified in 7 cases (70%), while other findings were less frequent. GLUT1 immunohistochemical staining gave negative results in all cases. No frequency differences were observed between histological characteristics and different subtypes, and no correlations were found with complications or atypical evolution (p > 0.05).
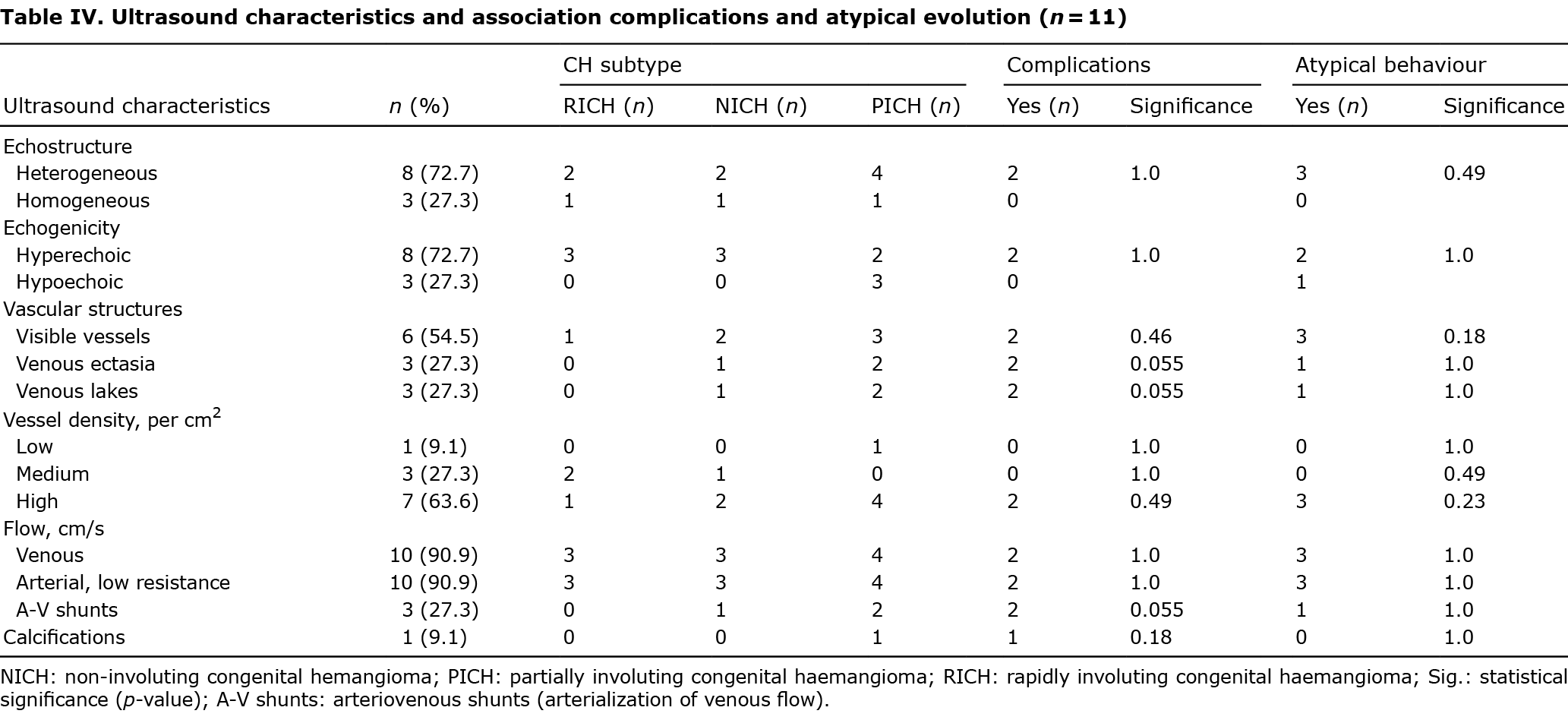
Eleven patients had undergone at least 1 ultrasound scan. The ultrasound characteristics of this subsample are shown in Table IV. Most lesions were heterogeneous (n = 8, 72.7%) and hyperechoic (n = 7, 72.7%) in relation to muscle. Visible vessels were detected in 6 lesions, of which 3 showed vascular ectasia and venous lakes (Fig. 1C). High vascular density was observed in 63.6% of cases, and venous flow and low resistance arterial flow in 90.9%. Three CHs (27.3%) had arteriovenous microshunts. Calcifications (phleboliths) were found in only 1 case. No CHs had high-resistance arteries (the highest detected flow velocity was 70 cm/s) or thrombi. In addition to these findings, in 1 patient a feeding vessel with arterial flow arising from the epigastric and common femoral arteries was observed (patient 12). A tendency towards significance was found in the association of vascular ectasia, venous lakes and arteriovenous microshunts with the occurrence of complications (p = 0.055).
In patient 13 (PICH with high-output heart failure, mild bleeding and ulceration), ultrasound showed vascular ectasia, venous lakes, calcifications and arteriovenous microshunts (Fig. 1). An MRI scan was deemed necessary in this case, and the images showed a new feeding artery arising from the deep femoral artery, and prominent draining veins leading to the external iliac vein.
Four patients (22.2%) underwent an echocardiogram. CH was the justification for this test in only 1 patient, in whom the results showed no signs of heart failure. The child showing signs of high-output heart failure related to the haemangioma (patient 13) was sent for an echocardiogram because a murmur had been detected on auscultation. This patient’s heart shrank gradually, reaching normal size at 6 months.
Two children (11.1%) received treatment: in patient 1, topical timolol was applied, but achieved no response; and in patient 7 (retroauricular NICH with late expansion) (Fig. 3), the lesion was excised after unsuccessful treatment with propranolol.
DISCUSSION
This study presents the clinical, histological and ultrasound characteristics of a series of 18 patients with CH. Very few publications have reported on all of these aspects while also analysing their association with the occurrence of complications and atypical behaviour. Important findings of the current study include the association between CH size and complications, and the higher frequency of complications in CHs with ectasia, venous lakes and microshunts.
Unlike infantile haemangioma, CH is not associated with prematurity or female sex; indeed, the literature is inconclusive on associations with either sex (3, 8, 11, 13–15). In the current study series, only 1 child was born before week 37, and most patients were boys. The most common reported locations of CH are the extremities, followed by the trunk and head and neck area (8, 13–16), as in the current study.
CHs arise and develop in the uterus and are fully developed at birth. In PICH and RICH, partial or complete involution occurs after birth, while in NICH, this process is believed to end during foetal development (3, 4). According to Nasseri et al. (3), the clinical features of PICH are indistinguishable from those of NICH or RICH (3, 13, 14, 16). In the current study patients, complete or partial involution time ranged from 3 to 24 months, with a mean value of almost 12 months. Similarly, the literature describes involution times of between 4 and 31 months of age (3, 8, 13, 14).
In recent years, some authors have described cases of CH with atypical features, including pain, segmental presentation (11, 17), increased vascular markings or expansion (11, 18, 19). Vascular changes manifest as increased telangiectasia or venous prominence and the formation of papules or pyogenic granulomas on the surface (11, 18, 19). Reports of CHs that increase in size in the months or years after birth (12, 18–20) have led some authors to propose the term tardive expansion congenital haemangioma (TECH) (12). Atypical changes may be associated with the occurrence of thrombocytopaenia and localized coagulopathy, and have mainly been described in NICH and PICH. West et al. (11) recommend blood tests in these cases. In the current series, the 5 patients who showed atypical features had NICH or PICH. Tardive expansion (TECH) was observed in 2 lesions: 1 located in the preauricular region and the other on the abdomen. This finding is coherent with the literature, which describes a predilection of TECH for the head and trunk (12, 18). The abdominal lesion had a feeding artery, which is common in TECH (12). No abnormal blood test results were observed in either patient.
It was previously thought that different CH subtypes tended to show specific histological features (2, 5, 7), such as calcifications, hemosiderin or thrombosis in RICH; and large lobules, wider lumina and arteriovenous fistulas in NICH. In a large series of patients, El Zein et al. (14) recently observed 4 different lobule patterns and a high frequency of extralobular lymphatic vessels. Three lobule patterns were detected, the most frequent consisting of medium-sized capillaries with round distinct lumina, but the scleronodular pattern was not observed in any case. A high frequency of apparently lymphatic-like vessels outside the lobules was also recorded, although immunohistochemical staining was not used. Other findings (presence of hemosiderin, calcifications or thrombosis) were uncommon. No differences were found in frequency between clinical subtype and any histological finding. Like El Zein et al. (14), we consider that at least 1 year of follow-up is required to determine CH subtype.
Ultrasound is useful for distinguishing CH from other vascular abnormalities. Although sonographically similar to infantile haemangioma, CH has some distinctive features, such as well-defined tubular vascular structures, calcifications, large veins and arteriovenous microshunts (8, 21). As in previous studies, most of the current cases were heterogeneous and more than half of the lesions had visible vessels. Ectasia and venous lakes were found in 3 CHs, microshunts in 3 and phleboliths in 1.
CH can lead to mild complications, such as ulceration and bleeding, or more severe complications, such as heart failure or haematological alterations (transient coagulopathy or thrombocytopaenia). Serious complications usually manifest in the peripartum period and can be life-threatening, usually requiring monitoring and treatment (9, 10, 22, 23). Four patients in the current series had complications. One developed thrombocytopaenia in the first days of life and another showed haemodynamic alterations, with an echocardiogram showing left heart chamber dilation at 2 months of age. Unlike in previous studies, these alterations had no clinical repercussion and resolved spontaneously over the follow-up without the necessity for cardiovascular support.
Although progress has been made in understanding these tumours, no clear recommendations are in place for detecting complications early. Haematological and cardiological complications have been recorded mainly in children with large CHs (> 5 cm) (9, 10, 22, 23). The current study supports this trend, showing a significant relationship between the occurrence of complications and large CHs. Waelti et al. (15) found a significant association between the presence of venous lakes and the occurrence of heart failure, and a trend towards significance in the association of arteriovenous microshunts with heart failure, and in the association of ectasia and venous lakes with bleeding and ulceration. Similarly, the current study found that the presence of ectasia, venous lakes and microshunts showed a borderline association with the occurrence of complications. Therefore, we consider that echocardiography and blood testing is indicated in large CHs, or in cases in which ultrasound reveals ectasia, venous lakes or microshunts.
Despite the limitations of the current study, such as its retrospective nature, its small sample size, worse resolution of older ultrasound scanners that could lead to an unavoidable uncertain bias, and the lack of genetic testing, we consider that these findings can be extrapolated to other children with CHs, as we have confirmed certain observations published in the literature.
In conclusion, in the current series of 18 patients with CH, a significant relationship between CH size and the occurrence of complications was found. In addition, there was a borderline association between complications and the ultrasound findings of ectasia, venous lakes and arteriovenous microshunts. No specific clinical, histological or ultrasound characteristics were associated with any clinical subtype or with atypical behaviour. In view of the findings of the current study and those of previous studies, we consider that diagnosis of CH should be based primarily on clinical presentation, with the help of a biopsy or ultrasound scan; that clinical subtype should be defined by clinical course alone; that large CHs warrant haematological and cardiological assessment as they are more likely to lead to complications; and that when venous ectasia, venous lakes or microshunts are present these tests should also be considered.
ACKNOWLEDGEMENTS
We thank Julia May Turner for translating the manuscript into English and copy-editing the text. She has no conflicts of interest to disclose.
Institutional Review Board (IRB) exempted this investigation from review since this study was performed as a subanalysis of a larger project about infantile haemangiomas (entitled “Pediatric teledermatology. Healthcare impact with special relevance of the approach to infantile haemangiomas”) was approved by the local ethics committee of Alicante University General Hospital (Alcante, Spain).
Informed consent was obtained from parents to perform clinical imaging. This retrospective research was performed in accordance with the principles of the Declaration of Helsinki about medical research involving human subjects and the Declaration of Taipei regarding research on health databases, big data and biobanks.
The data reported in this study come from routine clinical practice. Thus, we consider informed consent from parents was not necessary for publication.
The authors have no conflicts of interest to declare.
REFERENCES
- Boon L, Enjolras O, Mulliken JB. Congenital hemangioma: evidence of accelerated involution. J Pediatr 1996; 52: 13837–13866.
- Enjolras O, Mulliken JB, Boon LM, Wassef M, Kozakewich HPW, Burrows PE. Noninvoluting congenital hemangioma: a rare cutaneous vascular anomaly. Plast Reconstr Surg 2001; 107: 1647–1654.
- Nasseri E, Piram M, McCuaig CC, Kokta V, Dubois J, Powell J. Partially involuting congenital hemangiomas: a report of 8 cases and review of the literature. J Am Acad Dermatol 2014; 70: 75–79.
- Mulliken JB, Enjolras O. Congenital hemangiomas and infantile hemangioma: missing links. J Am Acad Dermatol 2004; 50: 875–882.
- Berenguer B, Mulliken JB, Enjolras O, Boon LM, Wassef M, Josset P, et al. Rapidly involuting congenital hemangioma: clinical and histopathologic features. Pediatr Dev Pathol 2003; 6: 495–510.
- Goh SGN, Calonje E. Cutaneous vascular tumours: an update. Histopathology 2008; 52: 661–673.
- Krol A, MacArthur C. Congenital hemangiomas: rapidly involuting and noninvoluting congenital hemangiomas. Arch Facial Plast Surg 2016; 35: 124–127.
- Gorincour G, Kokta V, Rypens F, Garel L, Powell J, Dubois J. Imaging characteristics of two subtypes of congenital hemangiomas: rapidly involuting congenital hemangiomas and non-involuting congenital hemangiomas. Pediatr Radiol 2005; 35: 1178–1185.
- Baselga E, Cordisco MR, Garzon M, Lee MT, Alomar A, Blei F. Rapidly involuting congenital haemangioma associated with transient thrombocytopenia and coagulopathy: a case series. Br J Dermatol 2008; 158: 1363–1370.
- Weitz NA, Lauren CT, Starc TJ, Kandel JJ, Bateman DA, Morel KD, et al. Congenital cutaneous hemangioma causing cardiac failure: a case report and review of the literature. Pediatr Dermatol 2013; 30: e180–e190.
- West ES, Totoraitis K, Yadav B, Kirkorian AY, Drolet BA, Teng JM, et al. Atypical presentations of congenital hemangiomas: extending the clinical phenotype. Pediatr Dermatol 2019; 36: 835–842.
- Hua C, Wang L, Jin Y, Chen H, Ma G, Gong X, et al. A case series of tardive expansion congenital hemangioma: a variation of noninvoluting congenital hemangioma or a new hemangiomatous entity? J Am Acad Dermatol 2021; 84: 1371–1377.
- Braun V, Prey S, Gurioli C, Boralevi F, Taieb A, Grenier N, et al. Congenital haemangiomas: a single-centre retrospective review. BMJ Paediatr Open 2020; 4: e000816.
- El Zein S, Boccara O, Soupre V, Vieira AF, Bodemer C, Coulomb A, et al. The histopathology of congenital haemangioma and its clinical correlations: a long-term follow-up study of 55 cases. Histopathology 2020; 77: 275–283.
- Waelti SL, Rypens F, Damphousse A, Powell J, Soulez G, Messerli M, et al. Ultrasound findings in rapidly involuting congenital hemangioma (RICH) – beware of venous ectasia and venous lakes. Pediatr Radiol 2018; 48: 586–593.
- Larralde M, Solé JJ, Luna PC, Mosquera T, Abad ME. Hemangiomas congénitos rápidamente involutivos. Serie de 25 casos. Arch Argent Pediatr 2014; 12: 61–65.
- Smith RJ, Metry D, Deardorff MA, Heller E, Grand KL, Iacobas I, et al. Segmental congenital hemangiomas: three cases of a rare entity. Pediatr Dermatol 2020; 37: 548–553.
- Cossio ML, Dubois J, McCuaig CC, Coulombe J, Hatami A, Marcoux D, et al. Non-involuting congenital hemangiomas (NICH) with postnatal atypical growth: a case series. Pediatr Dermatol 2019; 36: 466–470.
- Knöpfel N, Wälchli R, Luchsinger I, Theiler M, Weibel L, Schwieger-Briel A. Congenital hemangioma exhibiting postnatal growth. Pediatr Dermatol 2019; 36: 548–549.
- Boix-Vilanova J, Baselga E, Vera A, Gonzalez-Hermosa M del R, Azaña JM, Martin-Santiago A. Expanding the phenotypes of congenital hemangiomas. Pediatr Dermatol 2020; 37: 872–876.
- Esposito F, Ferrara D, Di Serafino M, Diplomatico M, Vezzali N, Giugliano AM, et al. Classification and ultrasound findings of vascular anomalies in pediatric age: the essential. J Ultrasound 2019; 22: 13–25.
- Cohen-Cutler S, Szymanski LJ, Bockoven C, Miller JM, Moke D, Anselmo DM, et al. Catastrophic congenital hemangioma with severe coagulopathy leading to fatal cardiac failure: case report and review. Pediatr Dermatol 2021; 38: 1276–1282.
- Shah SS, Snelling BM, Sur S, Ramnath AR, Bandstra ES, Yavagal DR. Scalp congenital hemangioma with associated high-output cardiac failure in a premature infant: case report and review of literature. Interv Neuroradiol 2017; 23: 102–106.