ORIGINAL REPORT
Recent Changes in the Incidence and Characteristics of Cutaneous Squamous Cell Carcinomas in Finland from 2006 to 2020: A Retrospective Cohort Study
Marika LOUNAS1,2, Leea YLITALO1,2, Teea SALMI1,2, Juha JERNMAN3, Johanna PALVE2,4, Tiina LUUKKAALA5 and Niina KORHONEN1,2
1Department of Dermatology, Tampere University Hospital, Tampere, 2Faculty of Medicine and Health Technology, Tampere University, Tampere, 3Department of Pathology, Tampere University and Fimlab Laboratories, Tampere, 4Department of Plastic Surgery, Tampere University Hospital, Tampere, and 5Research, Development and Innovation Centre, Tampere University Hospital and Health Sciences, Faculty of Social Sciences, Tampere University, Tampere, Finland
Registers recording only 1 tumour per patient do not enable assessment of the real burden of cutaneous squamous cell carcinoma. To investigate recent changes in the incidence and characteristics of tumours, a retrospective 15-year patient cohort study was performed in Finland. Histopathological diagnoses of cutaneous squamous cell carcinomas diagnosed between 2016 and 2020 were obtained from the pathology database and clinical data from patient medical records and combined with previously collected data for the years 2006–2015. Altogether 1,472 patients with 2,056 tumours were identified. The crude incidence increased from 19/100,000 persons in 2006 to 42 in 2020 (p < 0.001), increasing most in people aged over 80 years. The percentage of tumours located on the trunk increased from 5.3% during the first 5-year period, 2006–2010, to 9.0% in 2016–2020. Also, the location of tumours was significantly different between men and women, as men had more tumours on the scalp and ears, and women on the lower limbs. A slight change in the tumours from poorly to well differentiated and a decrease in the invasion depth were noted between 2006 and 2020. As the burden of tumours continues to increase, more attention should be paid to their prevention.
SIGNIFICANCE
Cutaneous squamous cell carcinoma is the second most common skin cancer. To obtain a more accurate number of tumours in recent years, we performed a retrospective 15-year patient cohort study. The results showed that the incidence of these tumours is still increasing, especially in the older population. Even though most of the tumours were facial, tumours located on the trunk became increasingly common with time. Tumour locations were different between men and women, as men had more tumours on the scalp, ears, and trunk. Also, a slight change in tumour characteristics was noted towards better differentiation and shallower invasion depth.
Key words: cohort study; cutaneous squamous cell carcinoma; incidence; keratinocyte carcinoma; non- melanoma skin cancer; real-world incidence.
Citation: Acta Derm Venereol 2024; 104: adv39891. DOI https://doi.org/10.2340/actadv.v104.39891.
Copyright: 2024 © The Author(s). Published by MJS Publishing, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/).
Submitted: Mar 3, 2024; Accepted: Apr 24, 2024; Published: May 30, 2024
Corr: Marika Lounas, Department of Dermatology, Tampere University Hospital, Tampere/Faculty of Medicine and Health Technology, Tampere University, Teiskontie 35, FIN-33521 Tampere, Finland. E-mail: marika.lounas@tuni.fi
Competing interests and funding: The authors have no conflicts of interest to declare.
This study was supported financially by the Finnish Dermatological Society, Tampere University Hospital Support Foundation, Tampere University Hospital/ Project No. MK364, Competitive State Research Financing for the Expert Responsibility area of Tampere University Hospital /Project No. 9AC053 and the Finnish Cultural Foundation (grants awarded to Dr Lounas). These organizations were not involved in any way in the study design, the collection, analysis or interpretation of the data, the writing of the manuscript, or the decision to submit the manuscript for publication.
INTRODUCTION
Cutaneous squamous cell carcinoma (cSCC) is the second most common keratinocyte skin cancer (1) and the numbers of these tumours are reported to be increasing worldwide (2, 3). The currently available statistics may be an underestimation, however, as in most epidemiological studies only the patient’s first tumour or only 1 tumour per patient per year will normally have been taken into account (3–5). In reality, a single patient may have multiple cSCC tumours simultaneously or in the course of his or her lifetime (2, 6).
CSCCs cause a significant burden for both society and the affected individual. The costs associated with skin cancers are significant and continue to increase (7). In particular, hospital admissions and medication for advanced skin cancers contribute to the costs, while drug prescriptions account for almost half of the costs in advanced cSCC cases (8). For patients, cSCC and its treatment cause notable inconvenience and also cosmetic problems, because most of the tumours are located in the facial area (3, 9). Functional and psychosocial problems are also common, especially after treatment for metastatic cSCC (10).
We have reported previously that the incidence of cSCC in a patient cohort in Finland increased between 2006 and 2015 (2). Some clarification is needed, however, as to whether the incidence has continued to increase in recent years. Furthermore, there are limited data regarding changes in the incidence figures when each tumour is taken into account separately and also information on changes in tumour characteristics is scarce. The aim of the present work, therefore, was to examine developments in the incidence of cSCCs in the same region of Finland up to the end of the year 2020 and to analyse changes in the clinical and histological characteristics of the tumours.
MATERIALS AND METHODS
Study protocol
Histopathological diagnoses of “cutaneous squamous cell carcinoma” recorded between 1 January 2016 and 31 December 2020 were searched for in the pathology database of Fimlab, which is a provider of laboratory services in the Pirkanmaa region of Finland. The keywords “skin and cutaneous squamous cell carcinoma” and “carcinoma, squamous cell” were used. Only diagnoses of invasive cutaneous squamous cell carcinoma were accepted. In situ cSCC, non-primary cSCC and samples with an uncertain diagnosis were excluded. If the same patient had multiple primary tumours, each of them was taken into account as an independent instance.
The medical records of all the cSCC patients identified in this way were recovered from Tampere University Hospital, the largest hospital in the Pirkanmaa region and its tertiary referral centre. Detailed data on each primary cSCC case were obtained by reviewing both the patient medical records and the histopathological reports. The results of biopsies and excisions were combined for each cSCC tumour individually.
The data on demographic factors included the patient’s age at diagnosis of the primary tumour, sex, and immunosuppression, and the tumour data collected from the patient records or histopathological reports comprised the anatomical localization of the tumour, its degree of differentiation, and the depth of invasion. Tumours were assigned to one of the following 10 anatomical sites: lip, eyelid, ear, face, neck/scalp, trunk, upper extremity, lower extremity, anogenital area, or oral cavity, based on the patient’s medical records. The clinical diameter of the tumour was included if it was reported with sufficient precision in the medical records. In cases where it was suspected that the clinical diameter may have been estimated and not measured, the diameter was excluded from the analysis. In addition, if the patient’s medical records contained a prior history of premalignant skin lesions or other skin cancers in addition to cSCC or current treatment for such before the end of the period defined here, these data were included in the study. The premalignant skin lesions included were actinic keratosis and/or Bowen’s disease and the skin cancers recorded were basal cell carcinoma or cutaneous melanoma. Since the data were collected in exactly the same way as in our previous study (2), we were able to combine the newly collected data with the information collected previously to form a 15-year study period extending from 1 January 2006 to 31 December 2020. The only exception was that perineural invasion (PNI), the tumour subtype, and the clinical diameter of the tumour were observed only in the most recent years, 2016–2020.
The institutional review board of Tampere University Hospital, Finland, approved this retrospective study.
Statistical analysis
The clinical data and demographic variables for the cSCCs were evaluated using descriptive statistics and frequency tabulation. The data were examined year by year and divided into three 5-year periods. Annual population figures for the Pirkanmaa region were obtained from Statistics Finland for use in the incidence calculations. The population of Pirkanmaa in the last year of the study, 2020, was 522,852. (11) Annual incidence rates were expressed as the number of cSCC cases per 100,000 of population. The numerator of the incidence rate was the number of cSCC cases diagnosed in each calendar year, independently of the number of patients. Patients could be counted more than once in a year if they had more than 1 primary tumour during that time.
Categorical data were described by the number of patients with percentages. Differences between the categorical variables were tested using Pearson’s χ2 test or Fisher’s exact test. Due to the skewed distributions, continuous variables were shown by medians with interquartile ranges, or else ranges and differences between the two distributions were tested by Mann–Whitney test. Statistical analyses were performed using IBM SPSS Statistics for Windows, Version 26.0 software (IBM Corp, Armonk, NY, USA). The statistical significances of trends in annual numbers were tested with the χ2 test, those of trends in proportions by RStudio version 1.4.1103 (R Foundation for Statistical Computing, Vienna, Austria; https://www.R-project.org/). Two-sided p-values under 0.05 were considered statistically significant.
RESULTS
After excluding 70 cases that did not meet the inclusion criteria and 448 samples due to diagnostic duplication (i.e., biopsies and final excisions of exactly the same cSCC tumours), altogether 698 patients with 925 cSCC tumours diagnosed between 2016 and 2020 were identified, which meant that a total of 1,472 patients with 2,056 cSCC tumours were observed during the entire period 2006–2020 (Table I). The number of tumours per patient ranged from 1 to 28.
| Factor | n (%) |
| Sex, n (%) | |
| Men | 791 (53.7) |
| Women | 681 (46.3) |
| Age, years, n (%) | |
| < 60 | 60 (4.1) |
| 60–69 | 147 (10.0) |
| 70–79 | 458 (31.1) |
| 80–89 | 607 (41.2) |
| > 90 | 200 (13.6) |
| Immunosuppression, n (%) | 151 (10.3) |
| Heart transplant | 10 |
| Kidney transplant | 39 |
| Liver transplant | 6 |
| Rheumatoid arthritis | 43 |
| Leukaemia | 24 |
| Lymphoma | 24 |
| Othersa | 5 |
| Actinic keratosis and/or Bowen’s disease*, n (%) | 1047 (71.1) |
| Basal cell carcinoma*, n (%) | 558 (37.9) |
| Cutaneous melanoma*, n (%) | 91 (6.2) |
| aTreatment with BRAF inhibitor or lung transplant. *Patients were indicated as having actinic keratosis, Bowen’s disease, basal cell carcinoma, or cutaneous melanoma if any prior history or current treatment of such a lesion was recorded in the clinical notes before the end of the period studied here. | |
The annual number of cSCC tumours increased from 88 in 2006 to 220 in 2020, and similar increases were observed in the numbers of men and women (51 to 135 in men and 37 to 85 in women, p < 0.001 in all the analyses). The crude incidence increased from 19 per 100,000 persons in 2006 to 42 in 2020 (p < 0.001) (Fig. 1), and an increasing trend was found in both men (21.9 to 52.4) and women (15.2 to 32.0) during the same period (p < 0.001). During the more recent period, 2016–2020, the age-specific incidence increased especially markedly in the groups aged 80 years or over (Fig. 2). The median age of the cSCC patients at the time of the first tumour in the same period was 80 years (interquartile range 73–86) for men and 83 (IQR 75–88) for women. The median age of the women was also 83 between 2006–2015 but only 78 in men (2), although this age difference in the men was not statistically significant.
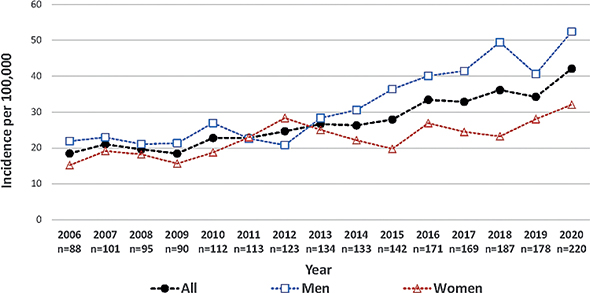
Fig. 1. Annual number and crude incidence (per 100,000) of cutaneous squamous cell carcinoma tumours, 2006–2020 (n = 2,056).
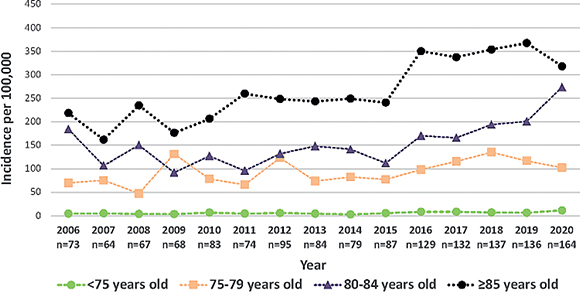
Fig. 2. Annual incidence (per 100,000) of cutaneous squamous cell carcinoma patients by age groups, 2006–2020 (n = 1,472).
A total of 10.3% of the patients during 2006–2020 were considered immunosuppressed, the 2 most common reasons for this being solid organ transplantation and rheumatoid arthritis. Other reasons taken into consideration were lymphoma, leukaemia, HIV, and treatment with a BRAF-inhibitor. Over 2/3 of the patients had a previous or current premalignant lesion, i.e., actinic keratosis and/or Bowen’s disease, besides cSCC, every third cSCC patient had a basal cell carcinoma and 6.2% of the patients had a cutaneous melanoma (Table I).
The most common location of cSCC during all the 5-year periods was the face (excluding ears, eyelids, and lips), so that during 2016–2020 as many as 46.2% of the tumours (427 out of 925) were located in the facial area. During the years 2006–2015 the second most common location was an upper limb while during 2016–2020 it was the scalp and neck (n = 108, 11.7%). The third most common location in 2016–2020 was an ear (n = 96, 10.4%), as also in 2006–2015. The number of cSCC tumours on the trunk increased in both men and women over time, whereas the numbers of tumours on a lip or an upper limb decreased (Table II).
There was a significant difference between the men and women in the location of the tumours over the whole period 2006–2020. Between 2016 and 2020 the most significant difference was in tumours located in the ear, with men having 89.6% there and women 10.4%. There was also a significant sex difference in scalp and neck tumours, the men having 79.6% of these and the women 20.4%. In total, 69.9% of the tumours located on the trunk were in men and 30.1% in women (Fig. 3).
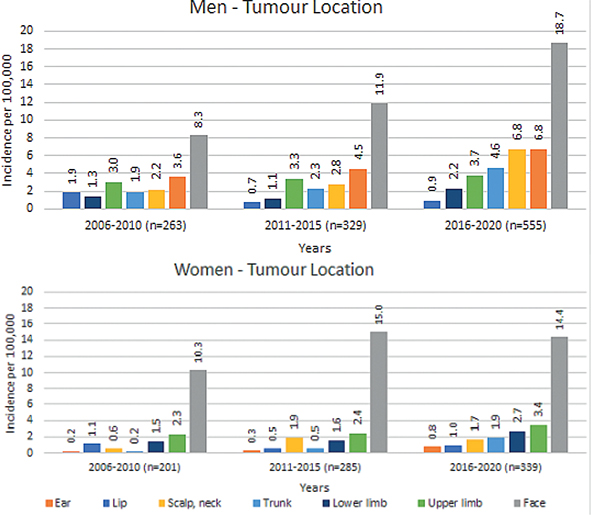
Fig. 3. Incidence of cutaneous squamous cell carcinoma tumours in 2006–2020, by location (eyelids, anogenital area, and oral cavity excluded), calculated separately for men and women.
Comparing the characteristics of the tumours between 5-year periods, the majority of cSCCs (48.2%) were well differentiated between 2016 and 2020 but the number of poorly differentiated tumours was shown to decrease. Most tumours had an invasion depth of less than 2mm in each 5-year period and the percentage of tumours with an invasion depth of over 4mm decreased from 9.7% in the first period to 8.4% in 2016–2020. Perineural invasion and tumour diameters were only investigated in the interval 2016–2020, the outcome being that perineural invasion was present in 29 cases (3.1%), and 48.2% of the tumours were under 2.0 centimetres in diameter, with only 3.2% over 4.0 cm in diameter (Table II).
DISCUSSION
The results showed that the numbers and incidences of cSCCs in Finland were still increasing during the most recent period studied, 2016–2020, especially in people aged 80 years or older. Even though cSCCs were shown to have become more common in both sexes, the increased figures were more evident among men. In parallel to our findings, incidence rates have been shown previously to increase with age and be associated with male sex (12). An ageing population is a challenge faced by many countries, including Finland, so that where 23.3% of Finns were over 65 years old in 2022, it is estimated that by 2060 the percentage will be 30.9% (13). Ageing of the population combined with increasing incidence of cSCCs among older individuals will lead to a substantial increase in the number of cSCCs and subsequently to a growing need for healthcare services to treat skin cancers. Moreover, as the population ages the number of individuals with multiple medication is likely to increase (14), which could also potentially affect the incidence of cSCC. Clinicians should be aware of drugs that are associated with increased rates of skin cancer, such as cyclosporine and azathioprine, and carefully monitor the skin of patients who take these drugs (15).
The exact reasons for the rising trend in the numbers of cSCC cases remain unclear, but several factors are thought to be implicated. It appears that cumulative UV exposure predisposes individuals to the development of cSCCs (16) and most cSCCs arise in the context of actinic keratosis in patients with chronic photoaging. The mechanism of cSCC development is thought to be hyperproliferation of keratinocytes, which requires multiple gene mutations, making transformation into cSCC complicated, so that it often takes many years (12). The use of a sunscreen seems to reduce the risk of cSCC, (17) while β-human papillomavirus and smoking have been shown in previous studies to have the opposite effect. (12) Furthermore, it is known that people with immunosuppression have a greater risk of developing a cSCC (12,18) and that this may be associated with the duration of immunosuppression (15). Education for patients on photoprotection and also skin examinations are necessary in order to reduce the risk of skin cancers (19). A systematic review of the effects of behavioural counselling for skin cancer prevention showed that interventions can increase sun protection behaviour but, even so, the evidence on whether such interventions are associated with decreased sunburn frequency was not conclusive (20).
Limited data are available concerning the differences in the anatomical distribution of cSCC between the sexes (21), but the present results show that the men had more tumours on the scalp and ears during a 15-year follow-up, which is in line with previous findings (21, 22). One reason for this may be general differences in hairstyle and clothing that expose certain anatomical locations in men to more sunlight than in women. On the other hand, women had slightly more tumours on the lower legs, as already observed in other studies (21, 23). Different clothing and sunbathing habits compared with men might at least partly explain this but the role of repeated depilation of the lower extremities in causing chronic inflammation has also been speculated (21). Further studies are needed to investigate the reasons more carefully. In one Australian cohort the incidence of cSCC located on the trunk was higher in women than in men (22), but in our Finnish population the opposite was the case, as almost three-quarters of the trunk cSCCs were in men. The incidence of tumours on the trunk in our cohort increased with time, as has also been seen previously in a Norwegian study (23). Even though the majority of tumours were located on the face, there were also a significant number situated below the neck. It is therefore of the utmost importance that physicians allow themselves enough time to examine the whole body. This is particularly important during follow-up visits, especially if the patient has already had a premalignant or malignant lesion (24). The effectiveness of skin screening by a non-dermatologist needs to be studied more, but it seems at least that dermatologists find melanomas at an earlier stage than do general practitioners or the patients themselves (25). Finding a skin cancer at an early stage is crucial as it reduces the cost of treatment (26).
It is notable that a slight change was observed here from poorly to well-differentiated tumours. Almost half of the tumours were well differentiated, as also reported previously (27, 28), and the invasion depth was also slightly shallower in the recent data than in the previous period. Several tumour-related high-risk factors have been identified and are listed in European consensus-based interdisciplinary guidelines (12), so that the grade of tumour differentiation is one independent prognostic factor for overall survival and metastatic disease (27). Also, other features, such as male sex, presence of comorbidities, immunosuppression, and actinic keratosis, have been associated with keratinocyte cancer progression (29). Furthermore, the clinical size of a tumour is also one recognized prognostic factor, so that the TNM classification and several guidelines use clinical size as the main high-risk feature. It is therefore recommended to measure the tumour before excision (12). The tumour’s clinical size was stated accurately enough to be recorded in only 63.8% of cases in our cohort, leaving room for improvement. In general, it is important to get enough information on tumours in order to formulate a prognosis and decide on the best treatment. As there is no international consensus on the follow-up schedule for cSCC patients, schedules need to be planned individually based on intrinsic and extrinsic risk factors (30).
The strength of our study is that it is one of few to record each tumour individually, as we used histological and clinical records and were able to confirm the diagnosis reliably, to remove duplicates (if several samples were taken from one tumour), and to distinguish residual tumours from primary tumours in order to analyse time trends and the characteristics of the tumours.
Due to the retrospective nature of the study, it has some limitations. It was not possible to collect all the relevant information on each cSCC, and in some cases the clinical information was not accurate enough to be included. Moreover, it is possible that we may still have underestimated the burden of cSCCs, as a few of them may have been treated completely in the private sector and were thus not included in our cohort. In view of the nature of the Finnish healthcare system, however, and the national guidelines concerning cSCCs, we assume that the number of tumours treated outside the public health sector is low.
In conclusion, the incidence of cSCC is still increasing significantly, especially in older patients, and a slight rise in the proportion of tumours located on the trunk or lower limbs highlights the importance of a whole-body examination in order to find premalignant lesions and small tumours early enough to avoid extensive excisions. As the burden of cSCC increases, attention must be paid to providing adequate resources for the treatment and prevention of skin cancers.
ACKNOWLEDGEMENTS
IRB approval status: The institutional review board of Tampere University Hospital, Finland, approved this retrospective study.
REFERENCES
- Que S, Zwald F, Schmults C. Cutaneous squamous cell carcinoma: incidence, risk factors, diagnosis, and staging. J Am Acad Dermatol 2018; 78: 237–247.
- Korhonen N, Ylitalo L, Luukkaala T, Itkonen J, Häihälä H, Jernman J, et al. Characteristics and trends of cutaneous squamous cell carcinoma in a patient cohort in Finland 2006–2015. Acta Derm Venereol 2019; 99: 412–416.
- Lomas A, Leonardi-Bee J, Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol 2012; 166: 1069–1080.
- Tokez S, Hollestein L, Louwman M, Nijsten T, Wakkee M. Incidence of multiple vs first cutaneous squamous cell carcinoma on a nationwide scale and estimation of future incidences of cutaneous squamous cell carcinoma. JAMA Dermatol 2020; 156: 1300–1306.
- Wilson A, Goltsman D, Nankervis J, Clark J, Gupta R, Ashford B. Defining the incidence of cutaneous squamous cell carcinoma in coastal NSW Australia. Aust J Dermatol 2022; 63: 213–216.
- Schmults CD, Karia PS, Carter JB, Han J, Qureshi AA. Factors predictive of recurrence and death from cutaneous squamous cell carcinoma: a 10-year, single-institution cohort study. JAMA Dermatol 2013; 149: 541–547.
- Noels E, Hollestein L, Luijkx K, Louwman M, de Uyl-de Groot C, van den Bos R, et al. Increasing costs of skin cancer due to increasing incidence and introduction of pharmaceuticals, 2007–2017. Acta Derm Venereol 2020; 100: adv00147.
- Ronconi G, Piccinni C, Dondi L, Calabria S, Pedrini A, Esposito I, et al. Identification of cases and estimate of direct costs of unresectable and advanced cutaneous squamous cell carcinoma: real-world data from a large Italian database. Br J Dermatol 2020; 183: 172–174.
- Korhonen N, Ylitalo L, Luukkaala T, Itkonen J, Häihälä H, Jernman J, et al. Premalignant lesions, basal cell carcinoma and melanoma in patients with cutaneous squamous cell carcinoma. Arch Dermatol Res 2021; 313: 879–884.
- Wang AY, Palme CE, Wang JT, Morgan GJ, Gebski V, Gilchrist J, et al. Quality of life assessment in patients treated for metastatic cutaneous squamous cell carcinoma of the head and neck. J Laryngol Otol 2013; 127: S39–47.
- Official Statistics of Finland (OSF). Population structure. 2021. Helsinki: Statistics Finland [referred: Sept 6, 2023]. Available from: https://pxdata.stat.fi/PxWeb/pxweb/fi/StatFin/StatFin__vaerak/statfin_vaerak_pxt_11ra.px/table/tableViewLayout1/
- Stratigos AJ, Garbe C, Dessinioti C, Lebbe C, van Akkooi A, Bataille V, et al. European consensus-based interdisciplinary guideline for invasive cutaneous squamous cell carcinoma. Part 1: Diagnostics and prevention-Update 2023. Eur J Cancer 193:113251.
- Official Statistics of Finland (OSF). Population projection. 2021. Helsinki: Statistics Finland [referred: Sept 28, 2023]. Available from: http://www.stat.fi/til/vaenn/2021/vaenn_2021_2021-09-30_tie_001_fi.html.
- Pazan F, Wehling M. Polypharmacy in older adults: a narrative review of definitions, epidemiology and consequences. Eur Geriatr Med 2021; 12: 443–452.
- Bašić-Jukić N, Borlinić T, Tešanović D, Mokos I, Krešimir Lukić I, Bukvić Mokos Z. Risk factors for non-melanoma skin cancer development in renal transplant recipients: a 40 year retrospective study in Croatia. Croat Med J 2022; 63: 148–155.
- International Agency for Research on Cancer Working Group on artificial ultraviolet (UV) light and skin cancer. The association of use of sunbeds with cutaneous malignant melanoma and other skin cancers: a systematic review. Int J Cancer 2007; 120: 1116–1122.
- Waldman RA, Grant-Kels JM. The role of sunscreen in the prevention of cutaneous melanoma and nonmelanoma skin cancer. J Am Acad Dermatol 2019; 80: 574–576.
- Garrett GL, Blanc PD, Boscardin J, Lloyd AA, Ahmed RL, Anthony T, et al. Incidence of and risk factors for skin cancer in organ transplant recipients in the United States. JAMA Dermatol 2017; 153: 296–303.
- Morton SK, Harrison SL. Slip, slop, slap, slide, seek and sport: a systematic scoping review of sun protection in sport in Australasia. Curr Oncol 2022; 30: 401–415.
- Henrikson NB, Morrison CC, Blasi PR, Nguyen M, Shibuya KC, Patnode CD. Behavioral counseling for skin cancer prevention: evidence report and systematic review for the US Preventive Services Task Force. JAMA 2018; 319: 1143–1157.
- Kim Y, Feng J, Su KA, Asgari MM. Sex-based differences in the anatomic distribution of cutaneous squamous cell carcinoma. Int J Womens Dermatol 2020; 6: 286–289.
- Subramaniam P, Olsen CM, Thompson BS, Whiteman DC, Neale RE, for the QSkin Sun and Health Study Investigators. Anatomical distributions of basal cell carcinoma and squamous cell carcinoma in a population-based study in Queensland, Australia. JAMA Dermatol 2017; 153: 175–182.
- Robsahm TE, Helsing P, Veierød MB. Cutaneous squamous cell carcinoma in Norway 1963–2011: increasing incidence and stable mortality. Cancer Med 2015; 4: 472–480.
- Hoorens I, Vossaert K, Pil L, Boone B, De Schepper S, Ongenae K, et al. Total-body examination vs lesion-directed skin cancer screening. JAMA Dermatol 2016; 152: 27–34.
- Fisher NM, Schaffer JV, Berwick M, Bolognia JL. Breslow depth of cutaneous melanoma: impact of factors related to surveillance of the skin, including prior skin biopsies and family history of melanoma. J Am Acad Dermatol 2005; 53: 393–406.
- Rogers HW, Coldiron BM. A relative value unit-based cost comparison of treatment modalities for nonmelanoma skin cancer: effect of the loss of the Mohs multiple surgery reduction exemption. J Am Acad Dermatol 2009; 61: 96–103.
- Brinkman JN, Hajder E, van der Holt B, Den Bakker MA, Hovius SER, Mureau MAM. The effect of differentiation grade of cutaneous squamous cell carcinoma on excision margins, local recurrence, metastasis, and patient survival: a retrospective follow-up study. Ann Plast Surg 2015; 75: 323–326.
- Marsidi N, Ottevanger R, Bouwes Bavinck JN, Krekel-Taminiau NMA, Goeman JJ, Genders RE. Risk factors for incomplete excision of cutaneous squamous cell carcinoma: a large cohort study. J Eur Acad Dermatol Venereol 2022; 36: 1229–1234.
- Tuominen S, Ukkola-Vuoti L, Riihilä P, Knuutila JS, Kähäri VM, Lassenius M, et al. Retrospective, registry-based, cohort investigation of clinical outcomes in patients with cutaneous squamous cell carcinoma and basal cell carcinoma in Finland. Acta Derm Venereol 2022; 102: adv00693.
- Stratigos AJ, Garbe C, Dessinioti C, Lebbe C, van Akkooi A, Bataille V, et al. European consensus-based interdisciplinary guideline for invasive cutaneous squamous cell carcinoma: Part 2. Treatment-Update 2023. Eur J Cancer 2023; 193: 113252.