ORIGINAL REPORT
Mycosis Fungoides and Associated Malignancies in a Dutch Nationwide Retrospective Cohort Study
Rosanne OTTEVANGER1, Esther VERMAAS1, Rein WILLEMZE1, Anne-Roos SCHRADER2, Patty M. JANSEN2, Jelle J. GOEMAN3, Hein PUTTER3, Maarten H. VERMEER1 and Koen D. QUINT1
1Department of Dermatology, Leiden University Medical Center, Leiden, 2Department of Pathology, Leiden University Medical Center, Leiden, and 3Department of Statistics, Leiden University Medical Center, Leiden, the Netherlands
The prognosis of patients with mycosis fungoides is variable. As the current literature is scarce and shows mixed results this study investigates the incidence of other primary malignancies in mycosis fungoides patients. A retrospective, nationwide, population- based cohort study was performed with patients with mycosis fungoides between 2000 and 2020 in The Netherlands. All histopathology reports were requested from the Nationwide Network and Registry of Histo- and Cytopathology and screened for other primary malignancies. Lifelong incidence rates were used to compare the incidence of malignancies in mycosis fungoides patients and the general population. In total 1,024 patients were included with a mean follow-up of 10 years (SD 6). A total of 294 cases of other primary malignancies were found with 29% of the mycosis fungoides patients developing at least 1 other primary malignancy. Only cutaneous (odds ratio [OR] 2.54; CI 2.0–3.2) and haematological malignancies (OR 2.62; CI 2.00–3.42) had a statistically significant higher incidence than the Dutch population overall. Mycosis fungoides patients have a significantly increased risk of developing melanomas (OR 2.76; CI 2.11–3.59) and cutaneous squamous cell carcinomas mycosis fungoides (OR 2.34; CI 1.58–3.45). This study shows no association between mycosis fungoides and other solid organ tumours; however, such patients are significantly at risk of developing other haematological and cutaneous malignancies. Clinicians should be aware of this increased risk.
Key words: concomitant malignancies; cutaneous T-cell lymphoma; epidemiology; folliculotropic mycosis fungoides; mycosis fungoides.
SIGNIFICANCE
The prognosis of patients with mycosis fungoides is variable and potentially influenced by other primary malignancies. This comparative study of mycosis fungoides patients and the general Dutch population found that mycosis fungoides patients are 2.5 times more likely to develop cutaneous and other haematological malignancies compared with the general Dutch population. Clinicians should be aware of this increased risk when conducting total body skin inspections during check-ups. Furthermore, clinicians could have a low threshold for repeated laboratory tests and additional imaging in case of clinical suspicion of other malignancies.
Citation: Acta Derm Venereol 2024; 104: adv40065. DOI: https://doi.org/10.2340/actadv.v104.40065.
Copyright: © 2024 The Author(s). Published by MJS Publishing, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/).
Submitted: Feb 7, 2024; Accepted: Aug 28, 2024; Published: Sep 15, 2024
Corr: Rosanne Ottevanger, Department of Dermatology, Leiden University Medical Center, Albinusdreef 2, 2333 ZA Leiden, the Netherlands. E-mail: r.ottevanger@lumc.nl
Competing interests and funding: The authors have no conflicts of interest to declare.
This study was made possible by an unrestricted research grant from Kyowa Kirin B.V.
INTRODUCTION
Mycosis fungoides (MF) is the most common subtype of cutaneous T-cell lymphoma (CTCL) and accounts for 50% of all primary cutaneous lymphomas (1). Its incidence has increased significantly in the last 2 decades (2, 3). MF mostly affects older adults, particularly around the age of 55 to 60 years, and is more common in men than in women. Most patients are diagnosed with early-stage MF, which typically has an indolent clinical course with patches and plaques that may evolve into tumours and/or erythroderma over years. In later stages, MF might progress to lymph nodes and viscera (1). In the early-stages of MF, skin lesions can be very subtle and differentiation from more common inflammatory dermatoses can be challenging, leading to a diagnostic delay of up to 90 months (4, 5).
The prognosis of patients with MF depends on the stage at time of diagnosis and progressiveness of the disease. The 10-year disease-specific survival of early stage MF was 97–98% for patients with limited patch/plaque disease (<10% skin surface), 83% for patients with generalized patch/plaque disease (>10% of skin surface), 42% in the presence of tumour stage disease, and about 20% in the case of lymph node involvement (1).
Previous literature has shown a higher incidence of other primary malignancies (OPMs) in MF patients that may affect overall survival (6–12). It is well known that patients with MF are particularly at risk for other Hodgkin lymphomas and non-Hodgkin lymphomas (7, 12). In addition, vice versa, MF was the most common OPM associated with lymphomatoid papulosis (LyP), another CTCL subtype with a prevalence of 6.2% in a cohort of 504 patients with LyP (13). Other MF-associated OPMs that have been described in MF patients concern cutaneous malignancies, such as melanomas, and solid tumours like urinary, colon, breast, and prostate cancer (7).
However, most of the studies reporting OPM in MF have been conducted on relatively small cohorts and have shown conflicting results. Also, these studies mainly included OPMs that developed subsequent to the MF diagnosis and did not include prior malignancies. Because of the diagnostic delay of up to several years and the relatively high age at time of diagnosis of MF patients, a large proportion of potential OPMs were not taken into account with this approach. This study aims to investigate the incidence of OPMs in MF patients in the Netherlands and to identify whether these are associated with MF.
MATERIALS AND METHODS
Patient selection
A retrospective, nationwide, population-based cohort study was performed with data from the Dutch Cutaneous Lymphomas Registry (DCLR). From this registry, all patients with a diagnosis of classical MF or folliculotropic MF (FMF) between January 2000 and January 2020 were selected. All diagnoses were made according to the classification system of the World Health Organization – European Organization for Research and Treatment of Cancer (WHO-EORTC) by a panel of dermatologists and pathologists during one of the quarterly meetings of the Dutch Cutaneous Lymphoma group (1). For patients still alive at the end of the inclusion period, there was a minimal 2-year follow-up period until May 2022. This study was reviewed and approved by the institutional Medical Ethical Board under the number G20.070.
Confirmation and definition of associated primary malignancies
From the included MF (classical MF and FMF) patients, all histopathology reports were requested from the Nationwide Network and Registry of Histo- and Cytopathology in the Netherlands (PALGA) (14). PALGA is a registry that includes all histo- and cytopathological reports in the Netherlands. These data were linked to the included MF patients using the Dutch civil registration number. All selected reports were screened for diagnoses of other malignancies. Each unique diagnosis of a malignancy was counted once, including recurrences or metastasis. Benign tumours and pre-malignancies or in situ malignancies were excluded, except for ductal carcinoma in situ of the breast. In any case where an additional type of cutaneous lymphoma was found, this was checked with the DCLR for confirmation, and the diagnosis in the DCLR was used as the definitive diagnosis. In the subset analysis for counts of keratinocyte carcinoma in individual patients, every unique lesion was counted once, and basal cell carcinoma (BCC) and cutaneous squamous cell carcinoma (cSCC) were analysed separately.
Incidence of cancer in the Dutch population
To compare the incidence of malignancies in MF patients with the general population, the cancer incidence data in the Dutch population were obtained from the Netherlands Cancer Registry (NCR). The NCR registration provides the statistics on cancer in the Netherlands based on the WHO guidelines (15). Incidence of OPM per calendar year, based on sex and age, was obtained from the NCR. Also data on the general Dutch population size, based on calendar year, sex, and age, were obtained from Statistics Netherlands (CBS) (16).
OPMs found in the study cohort were categorized according to the NCR, which is based on the 10th International Classification of Diseases (15). This categorization was necessary to create categories with sufficient numbers to perform statistical analysis. Therefore, it could be that the true number of tumours within one category could be higher than 1, but was only scored dichotomously (present or not present). In cases where a patient developed breast cancer initially in the right breast and subsequently in the left breast, these were considered as one OPM. In contrast, additional analyses for different subtypes of skin cancer were performed. Using the NCR and CBS databases, the OPM risk was calculated for each patient. Due to a lack of a nationwide incidence data for cutaneous BCC, these were excluded from statistical analysis. The total number of OPMs per patients was also registered.
Statistical analysis
Statistical analysis was performed with IBM SPSS statistics 25 (IBM Corp, Armonk, NY, USA) and reported following the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) guidelines (17, 18). Continuous data were reported as mean with standard deviation (SD) and as median with interquartile range (IQR), as appropriate, and categorical data as number and percentage (%). Patient outcomes were corrected for each individual calendar year, sex, and age by using lifelong incidence rates based on the NCR and CBS data. Statistical significance was analysed using Pearson’s χ2 goodness-of-fit-test, as appropriate, to determine the odds ratios (ORs) and their 95% confidence intervals (CIs). To test whether OPMs were more frequently diagnosed prior to or after the diagnosis of MF a one-proportion Z-test was performed. A 2-sided p-value of < 0.05 was considered statistically significant.
RESULTS
The study population consisted of 1,024 patients, including 796 patients (78%) with classical MF and 228 patients (22%) with FMF, with a male to female ratio of 1.8 for the total group (Table I). The mean (SD) age at time of diagnosis was 60 years (17). The mean (SD) duration of follow-up was 10 years (6). Analysis identified 294 cases of MF patients with OPM (Tables II and III). In these cases, a total of 377 different tumours were found. From these, only the cutaneous and haematological malignancies had a statistically significant higher incidence than the Dutch population (Table II). In total, 294 patients (29%) developed at least 1 OPM and approximately 7% of patients developed 2 or more OPM (Fig. 1).
| Other primary malignancy | Before MF diagnosis (n) | After MF diagnosis (n) | Total, n (%) | OR | [CI] | p-value |
| Skin* | 34 | 54 | 88 (23.3%) | 2.54 | [2.02–3.19] | <0.001 |
| Cutaneous squamous cell carcinoma | 18 | 43 | 61 (16.2%) | 2.76 | [2.11–3.59] | <0.001 |
| Melanoma | 15 | 11 | 26 (6.9%) | 2.34 | [1.58–3.45] | <0.001 |
| Haematological | 43 | 18 | 61 (16.2%) | 2.62 | [2.00–3.42] | <0.001 |
| Digestive tract | 22 | 27 | 49 (13.0%) | 0.76 | [0.56–1.02] | 0.07 |
| Male genital organs | 28 | 20 | 48 (12.7%) | 1.22 | [0.90–1.64] | 0.21 |
| Breast | 26 | 14 | 40 (10.6%) | 1.35 | [0.96–1.89] | 0.09 |
| Urinary tract | 10 | 18 | 28 (7.4%) | 0.97 | [0.66–1.42] | 0.87 |
| Respiratory tract | 10 | 14 | 24 (6.4%) | 0.54 | [0.35–0.84] | 0.01 |
| Head and neck | 4 | 8 | 12 (3.2%) | 0.60 | [0.65–2.13] | 0.60 |
| Female genital organs | 8 | 3 | 11 (2.9%) | 1.10 | [0.58–2.06] | 0.77 |
| Primary location unknown | 0 | 7 | 7 (1.9%) | 1.11 | [0.53–2.35] | 0.78 |
| Bone, articular cartilage, and soft tissues | 4 | 1 | 5 (1.3%) | 2.18 | [0.91–5.25] | 0.08 |
| Endocrine glands | 2 | 0 | 2 (0.5%) | 1.64 | [0.41–6.56] | 0.49 |
| Eye | 1 | 0 | 1 (0.3%) | 1.68 | [0.24–11.95] | 0.60 |
| Central nervous system | 1 | 0 | 1 (0.3%) | 0.29 | [0.04–2.07] | 0.22 |
| Total | 193 | 184 | 377 | – | – | – |
| *Excluding one patient with a Merkel cell carcinoma that was included in the skin category but odds ratio (OR) was not calculated separately. Basal cell carcinomas were not included within this registration. In bold: mycosis fungoides-associated primary malignancies. | ||||||
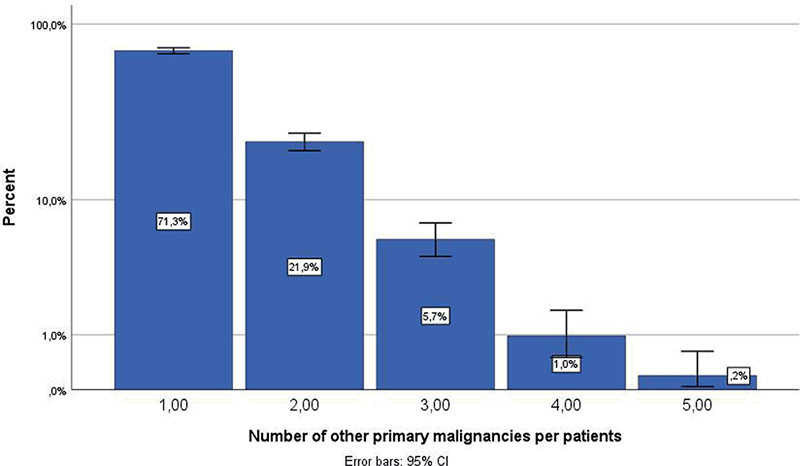
Fig. 1. Percentage of patients (n = 1,024) who had no other primary malignancy (OPM), at least 1 OPM, 2 OPMs, 3 OPMs, or 4 OPMs.
Cutaneous other primary malignancies
A total of 88 patients (23.3%) developed another cutaneous malignancy (excluding BCC). The MF patients had a statistically significant higher incidence of cutaneous OPM than the Dutch population, with an OR of 2.54 (95% CI 2.0–3.2). A malignancy-specific analysis showed that there was a significantly increased risk of developing melanomas (OR 2.76; 95% CI 2.11–3.59) and cSCC (OR 2.34; 95% CI 1.58–3.45). Within the cutaneous OPM, a diagnosis of cSCC was more prevalent after the MF diagnosis than before the MF diagnosis (p = < 0.001) (Table II). In melanomas, there was no difference in incidence prior to or after the diagnosis of MF (p = 0.43).
Haematological other primary malignancies and lymphoproliferative disorders
An overview of the haematological OPMs is presented in Table III. In total, 61 (16.1%) of the patients were diagnosed with another haematological malignancy and lymphoproliferative disorders. Compared with the overall Dutch population, there was a significantly increased risk of MF patients developing haematological malignancies and lymphoproliferative disorders with an OR of 2.62 (95% CI 2.00–3.42). More specifically, a statistically significant result was observed for LyP and Hodgkin lymphoma, with respective ORs of 76.22 (95% CI 50.35–115.32) and 6.28 (95% CI 2.02–19.55). Haematological OPMs and lymphoproliferative disorders were significantly more often diagnosed before than after the MF diagnosis (p = 0.001) (Table III).
Solid organ tumours
Solid tumours were not associated with an increased risk in MF patients compared with the general Dutch population risk (Table II). Patients with MF had a statistically decreased risk of lung cancer (OR 0.54; 95% CI 0.35–0.84, p = 0.01).
Risk factors
Sub-analysis did not show a statistically significant difference in the presence or type of OPM between male and female patients, or between classical MF and FMF patients.
Subgroup analysis of cutaneous malignancies
As presented in Table IV, subset analysis of keratinocyte carcinomas showed high numbers of BCCs and cSCCs present within 1 patient. In total, 16.6% of patients developed at least 1 unique BCC lesion and 6.0% developed at least 1 unique cSCC lesion. In total, 47.9% of MF patients (n = 81) developed multiple unique BCC lesions. Similarly, with cSCC, 47.5% (n = 29) developed more than 1 unique lesion.
DISCUSSION
In this study, the incidence of other malignancies was investigated in a large cohort of Dutch patients with MF before and after MF diagnosis with the aim of identifying MF-associated OPM. The results indicate that there was an association between MF and cutaneous and other haematological malignancies and lymphoproliferative disorders. In our research population, there were not only secondary, but also tertiary and even quaternity diagnoses of malignancies in MF patients.
The results of this study match with those observed in earlier studies, particularly for cutaneous and haematological malignancies and lymphoproliferative disorders (6–9, 11, 19–21). The observation of an increased incidence of LyP in this MF cohort mirrors the results of Melchers et al. (13), who found a high incidence of MF in LyP patients.
In cases of an increased risk of Hodgkin lymphoma and LyP, there are 2 likely explanations. Our results demonstrating that haematological malignancies are more prevalent before MF diagnosis suggest that a prior haematological malignancy can be a risk factor for developing MF later in life. It could be speculated that this is either due to an underlying molecular defect in a common hematopoietic stem cell or related to cytotoxicity from previous treatment for the haematological malignancy. As previous studies have described, this might be the result of the same neoplastic proliferation process (22). A non-random genetic event seems the most likely as MF and LyP have been shown to be clonally related (23).
The incidence of primary malignancies from other solid organ tumours was not increased in our cohort of MF patients. In addition, respiratory tract tumours were even significantly less prevalent This conflicts with previous studies that described an increased risk in cancers of the respiratory, urinary, and digestive tract (12). For example, Goyal et al. (12) found an association with lung cancer and MF with an incidence ratio of 8.3.
The different findings from these studies compared with this study could be explained by several factors. First, varying ethnic distributions with different a priori chances of certain tumour types were not taken into account in this study. Second, publication bias could also be a factor. Goyal et al. described an association of lung cancer and CTCL in their meta-analysis in 2021. In this analysis they first describe a statistical association, but after imputation of studies this was no longer statistically significant (12). Third, definitions of tumour types was not always described in previous studies. Therefore, analysis could be performed on different groups and also inclusion of potential pre-malignancies in these studies could not be ruled out. Last, and most importantly, frequent check-ups and more frequent diagnostic imaging because of the MF diagnosis could result in a higher rate of OPM for solid tumours in the MF group. However, the existence of national screening programmes for frequent solid organ tumours, such as colon and breast cancer, in the Netherlands, can be a major reason solid tumours were not more frequently found compared with previous studies. In addition, in the United States screening for lung cancer patients is advised for certain subgroups, which could also explain the discrepancy as related to respiratory tract tumours. The negative finding in this study that lung cancer is less prevalent does not mean that MF is a protective factor.
This study was unique for a number of reasons; it features a large national patient cohort based on clinicopathological confirmed cases of MF, MF-associated OPM determination with histological confirmation from a national high-quality database, long follow-up period, and statistical analysis based on lifelong incidence risk of the general population. Additionally, in contrast to most previous studies, this study included OPMs both before and after patients received their MF diagnosis.
No significant difference between MF and FMF, or between sexes in the development of MF-associated OPM was found, possibly indicating that these are not involved risk factors in MF-associated OPM development. This study did not have access to patient characteristics such as ethnicity, socioeconomic status, sun exposure, lifestyle, treatment, occupations, or family history. An interference of these potential risk factors cannot be ruled out.
Moreover, this research found that patients with MF who develop a non-melanoma skin carcinoma often develop more than 1 single lesion, which has also been previously described in the literature (24). Although there are no known BCC incidence rates in the Netherlands in the NCR, a recent Dutch publication showed that the estimated average BCC incidence rate was approximately 0.3%. In our study this was found in 17% of MF patients. It should be noted that, in this study population, nearly half of patients with MF developed more than 1 subsequent BCC lesion (25). The increased number of cutaneous malignancies could be explained by multiple factors. At first, more frequent total skin inspections by dermatologists could result in a higher incidence of cutaneous malignancies. Second, in particular post-diagnosis cSCC could be due to adverse effects of prescribed therapies like ultraviolet phototherapy (26). However, this study did not investigate the body distribution of cutaneous malignancies, which would also give further directions to the potential adverse effect of these therapies in relation to other risk factors such as sun exposure. However, the finding that SCCs were more often diagnosed after the MF diagnosis and melanomas were not is not per se an indication that this is related to the MF treatment as this could also be explained by the average age of SCCs (median 78 years) compared with melanomas (median 59 years) in relation to the MF (median age 55–60 years) (27–29).
A limitation of this study is the possibility of an underestimation of the risk patients have of developing an MF-associated OPM. This is because, in some cases, 1 patient developed multiple malignancies that fell into the same category but have only been scaled once. This risk was minimized by analysing individual malignancies in cutaneous and haematological categories. In this study we found a significantly decreased risk of lung cancer in MF patients compared with the Dutch population, but a reason for this could not be found. Because of the limited sample size due to the rarity of the disease compared with more prevalent cancers, other malignancies that showed increased, but not statistically significant, risk could speculatively still be clinically important.
An additional uncontrolled factor is that the OPM incidence rates of the study population were compared with the overall malignancy incidence rate of the general population, which was based only on age and gender, and could not be corrected for other risk factors (s comorbidities and smoking).
The findings of this study have a number of important implications for future practice. Clinicians should be aware of this increased risk and MF patients could be screened for potential signs of Hodgkin lymphomas, LyP, and other cutaneous malignancies, and therefore they should conduct regular total skin inspections and explore clinical signs of second malignancies. However, in contradiction to previous studies, which did find an association between MF and other solid organ tumours and suggest that there is a strong rationale for prospective screening studies, our study does not support these suggestions.
Clinicians could have a low threshold for repeated laboratory tests over time and additional imaging in case of clinical suspicion of other malignancies. However, the utility of regular screening by imaging and lab testing seems of low additional value but could require additional studies to confirm this hypothesis (30).
MF-associated primary malignancies are an important issue that should be further addressed by studying risk factors and causality. Underlying pathways are still unclear and need to be clarified so that in future practice MF-associated OPM can be diagnosed in an early stage and potentially increase surveillance during particular treatments of MF in order to reduce morbidity and mortality. Altogether, these results provide important insights into the incidence of MF-associated OPM and suggest a common biological pathway between haematological malignancies and MF.
In conclusion, our study shows that, in contrast to previous literature, there was no association between MF and other solid organ tumours. However, patients with MF are significantly at risk of developing other haematological and cutaneous malignancies compared with the general population. Clinicians should be aware of this increased risk when conducting total body skin inspections during routine check-ups. The higher risk of other hematologic malignancies suggests a potential common pathway of origin.
ACKNOWLEDGEMENTS
IRB approval status: This study was reviewed and approved by the institutional Medical Ethical Board of Leiden University Medical Center (G20.070).
REFERENCES
- Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH, et al. WHO-EORTC classification for cutaneous lymphomas. Blood 2005; 105: 3768–3785. https://doi.org/10.1182/blood-2004-09-3502
- Ottevanger R, de Bruin DT, Willemze R, Jansen PM, Bekkenk MW, de Haas ERM, et al. Incidence of mycosis fungoides and Sezary syndrome in the Netherlands between 2000 and 2020. Br J Dermatol 2021; 185: 434–435. https://doi.org/10.1111/bjd.20048
- Kaufman AE, Patel K, Goyal K, O’Leary D, Rubin N, Pearson D, et al. Mycosis fungoides: developments in incidence, treatment and survival. J Eur Acad Dermatol Venereol 2020; 34: 2288–2294. https://doi.org/10.1111/jdv.16325
- Alsayyah A. Is it mycosis fungoides? A comprehensive guide to reaching the diagnosis and avoiding common pitfalls. Ann Diagn Pathol 2020; 47: 151546. https://doi.org/10.1016/j.anndiagpath.2020.151546
- Scarisbrick JJ, Quaglino P, Prince HM, Papadavid E, Hodak E, Bagot M, et al. The PROCLIPI international registry of early-stage mycosis fungoides identifies substantial diagnostic delay in most patients. Br J Dermatol 2019; 181: 350–357. https://doi.org/10.1111/bjd.17258
- Cengiz FP, Emiroglu N, Onsun N. Frequency and risk factors for secondary malignancies in patients with mycosis fungoides. Turk J Haematol 2017; 34: 378–379. https://doi.org/10.4274/tjh.2017.0234
- Goyal A, O’Leary D, Goyal K, Rubin N, Bohjanen K, Hordinsky M, et al. Increased risk of second primary hematologic and solid malignancies in patients with mycosis fungoides: a Surveillance, Epidemiology, and End Results analysis. J Am Acad Dermatol 2020; 83: 404–411. https://doi.org/10.1016/j.jaad.2019.07.075
- Hodak E, Lessin S, Friedland R, Freud T, David M, Pavlovsky L, et al. New insights into associated co-morbidities in patients with cutaneous t-cell lymphoma (mycosis fungoides). Acta Derm Venereol 2013; 93: 451–455. https://doi.org/10.2340/00015555-1496
- Huang KP, Weinstock MA, Clarke CA, McMillan A, Hoppe RT, Kim YH. Second lymphomas and other malignant neoplasms in patients with mycosis fungoides and Sezary syndrome: evidence from population-based and clinical cohorts. Arch Dermatol 2007; 143: 45–50. https://doi.org/10.1001/archderm.143.1.45
- Lindahl LM, Fenger-Gron M, Iversen L. Subsequent cancers, mortality, and causes of death in patients with mycosis fungoides and parapsoriasis: a Danish nationwide, population-based cohort study. J Am Acad Dermatol 2014; 71: 529–535. https://doi.org/10.1016/j.jaad.2014.03.044
- Evans AV, Scarisbrick JJ, Child FJ, Acland KM, Whittaker SJ, Russell-Jones R. Cutaneous malignant melanoma in association with mycosis fungoides. J Am Acad Dermatol 2004; 50: 701–705. https://doi.org/10.1016/j.jaad.2003.11.054
- Goyal A, O’Leary D, Goyal K, Patel K, Pearson D, Janakiram M. Cutaneous T-cell lymphoma is associated with increased risk of lymphoma, melanoma, lung cancer, and bladder cancer. J Am Acad Dermatol 2021; 85: 1418–1428. https://doi.org/10.1016/j.jaad.2020.06.1033
- Melchers RC, Willemze R, Bekkenk MW, de Haas ERM, Horvath B, van Rossum MM, et al. Frequency and prognosis of associated malignancies in 504 patients with lymphomatoid papulosis. J Eur Acad Dermatol Venereol 2020; 34: 260–266. https://doi.org/10.1111/jdv.16065
- Casparie M, Tiebosch AT, Burger G, Blauwgeers H, van de Pol A, van Krieken JH, et al. Pathology databanking and biobanking in The Netherlands, a central role for PALGA, the nationwide histopathology and cytopathology data network and archive. Cell Oncol 2007; 29: 19–24. https://doi.org/10.1155/2007/971816
- IKNL [Internet]. Netherlands Comprehensive Cancer Organisation (IKNL) 2022. Available from: https://iknl.nl/home.
- Statistics Netherlands; Population on January 1st and average: sex, age, region. Central Bureau for Statistics, 2022. Available from: https://www.cbs.nl/.
- Corp. I. IBM SPSS Statistics for Windows. 25.0 ed. Armonk, NY; IBM Corp.; 2017.
- von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 370: 1453–1457. https://doi.org/10.1016/S0140-6736(07)61602-X
- Pielop JA, Brownell I, Duvic M. Mycosis fungoides associated with malignant melanoma and dysplastic nevus syndrome. Int J Dermatol 2003; 42: 116–122. https://doi.org/10.1046/j.1365-4362.2003.01697.x
- Miyatake J, Inoue H, Serizawa K, Morita Y, Espinoza JL, Tanaka H, et al. Synchronous occurrence of mycosis fungoides, diffuse large B cell lymphoma and acute myeloid leukemia. Intern Med 2018; 57: 1445–1453. https://doi.org/10.2169/internalmedicine.9668-17
- Scheu A, Schnabl SM, Steiner DP, Fend F, Berneburg M, Yazdi AS. Importance of diagnostics and risk of secondary malignancies in primary cutaneous lymphomas. J Dtsch Dermatol Ges 2021; 19: 373–381. https://doi.org/10.1111/ddg.14400
- Cieza-Diaz DE, Prieto-Torres L, Rodriguez-Pinilla SM, Cordoba Mascunano R, Manso Alonso R, Machan S, et al. Mycosis fungoides associated with lesions in the spectrum of primary cutaneous CD30+ lymphoproliferative disorders: the same process or 3 coexisting lymphomas? Am J Dermatopathol 2019; 41: 846–850. https://doi.org/10.1097/DAD.0000000000001423
- Chott A, Vonderheid EC, Olbricht S, Miao NN, Balk SP, Kadin ME. The dominant T cell clone is present in multiple regressing skin lesions and associated T cell lymphomas of patients with lymphomatoid papulosis. J Invest Dermatol, 1996; 106: 696–700. https://doi.org/10.1111/1523-1747.ep12345532
- Le K, Lim A, Samaraweera U, Morrow C, See A. Multiple squamous cell carcinomas in a patient with mycosis fungoides. Australas J Dermatol 2005; 46: 270–273. https://doi.org/10.1111/j.1440-0960.2005.00198.x
- Schreuder K, Hollestein L, Nijsten TEC, Wakkee M, Louwman MWJ. A nationwide study of the incidence and trends of first and multiple basal cell carcinomas in the Netherlands and prediction of future incidence. Br J Dermatol 2022; 186: 476–484. https://doi.org/10.1111/bjd.20871
- Reseghetti A, Tribbia G, Locati F, Naldi L, Marchesi L. Cutaneous malignant melanoma appearing during photochemotherapy of mycosis fungoides. Dermatology 1994; 189: 75–77. https://doi.org/10.1159/000246790
- Venables ZC, Autier P, Nijsten T, Wong KF, Langan SM, Rous B, et al. Nationwide incidence of metastatic cutaneous squamous cell carcinoma in England. JAMA Dermatol 2019; 155: 298–306. https://doi.org/10.1001/jamadermatol.2018.4219
- Shah R, Patel N, Patel Y, Toscani M, Barone J, Weber PF. Age demographics of subjects enrolled in global, interventional Phase 3 melanoma clinical trials. Ther Innov Regul Sci 2022; 56: 184–190. https://doi.org/10.1007/s43441-021-00362-0
- Jawed SI, Myskowski PL, Horwitz S, Moskowitz A, Querfeld C. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sezary syndrome): part I. Diagnosis; clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol 2014; 70: 205 e1–16; quiz 21–22. https://doi.org/10.1016/j.jaad.2013.07.049
- Goyal A, O’Leary D, Goyal K, Rubin N, Janakiram M. Screening for second malignancies in mycosis fungoides: non-Hodgkin lymphoma, Hodgkin lymphoma, lung cancer, bladder cancer and melanoma. J Eur Acad Dermatol Venereol 2021; 35: 1821–1829. https://doi.org/10.1111/jdv.17384