ORIGINAL REPORT
Short-term Heat Application Reduces Itch Intensity in Atopic Dermatitis: Insights from Mechanical Induction and Real-life Episodes
Joachim W. FLUHR1,2, Leonie HERZOG1,2, Razvigor DARLENSKI3,4, Tim MENTEL5 and Torsten ZUBERBIER1,2
1Charité – Universitätsmedizin Berlin, Institute of Allergology, Berlin, Germany, 2Fraunhofer Institute for Translational Medicine and Pharmacology ITMP, Allergology and Immunology, Frankfurt-am-Main, Germany, 3Department of Dermatology and Venereology, Acibadem City Clinic Tokuda Hospital, Sofia, Bulgaria, 4Department of Dermatology and Venereology, Medical Faculty, Trakia University, Stara Zagora, Bulgaria, and 5mibeTec GmbH, Brehna, Germany
Heat application is known to activate transient receptor potential (TRP) channels, which play a crucial role in sensory perception, including itch. In this study, the effect of a 5-s, 49°C heat application on itch intensity in atopic dermatitis (AD) patients was evaluated. The study comprised 2 parts: a controlled trial investigating the impact of brief heat treatment on mechanically induced itch, and a real-life study of AD patients experiencing itch attacks. A significant and immediate reduction in itch sensations following heat application was shown, with effects enduring over time. This response, however, showed notable individual variability, underscoring the potential of personalized approaches in AD treatment. Repeated applications of heat showed no habituation effect, suggesting its viability as a non-pharmacological, patient-tailored option for managing itch in AD. Further research in larger cohorts is warranted to refine treatment protocols and deepen understanding of the mechanisms involved.
SIGNIFICANCE
Heat can help relieve the itchiness experienced by people with a skin condition called atopic dermatitis. In our study, we looked at how applying a warm temperature (49°C) for just 5 s affected itchiness. We found that this brief heat application quickly and significantly reduced the feeling of itch, and the relief lasted for a while. However, the effectiveness of the heat treatment varied from person to person, which suggests that treatments could be customized for each individual’s needs. Importantly, using heat repeatedly still worked well, indicating that this could be a good non-drug treatment option for managing itch in people with atopic dermatitis.
Key words: pruritus; medical device; noxious heat; physical; epidermal barrier; eczema; epiivo.
Citation: Acta Derm Venereol 2024; 104: adv40127. DOI: https://doi.org/10.2340/actadv.v104.40127.
Copyright: © 2024 The Author(s). Published by MJS Publishing, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/).
Submitted: Feb 17, 2024; Accepted after revision: May 15, 2024; Published: Jun 17, 2024
Corr: Prof. Joachim Fluhr, Charité – Universitätsmedizin Berlin, Institute of Allergology, Campus Benjamin Franklin, Hindenburgdamm 30, Haus II, DE-12203 Berlin, Germany. E-mail: Joachim.fluhr@charite.d
Competing interests and funding: JWF and RD have received consulting fees from mibeTec; TM is an employee of mibeTec; LH and TZ have no conflict of interest in relation to this manuscript.
INTRODUCTION
Itch, an evolutionarily based defence mechanism against environmental stressors (pruritogens), reflects the sensation provoking a desire to scratch (1–3). In clinical settings, chronic itch lasts more than 6 weeks and can be caused by a plethora of dermatological (atopic dermatitis, psoriasis, infestations, urticaria), systemic, psychogenic, and neuropathic factors commonly overlapping in a single patient resulting in significant impairment of the disease-related quality of life (3–5). With a prevalence of 15–20% (6,7), chronic itch represents an active field of interdisciplinary research in the search for an effective therapeutic strategy and diagnostic outcomes (8, 9).
Skin cells like keratinocytes and fibroblasts react to external or internal itch-causing stimuli. These cells, along with immune cells such as macrophages, mast cells, and neutrophils, release various itch mediators. These mediators include serotonin, histamine, tryptase, thromboxanes, leukotrienes, nerve growth factor, tumour necrosis factor α, and ribonucleic acids (RNAs). They can activate neurons (pruriceptors). Activation of specific neurons (pruriceptors) leads to an increase in intracellular calcium. This increase is probably induced through at least 2 channels: transient receptor potential vanilloid 1 (TRPV1) and transient receptor potential ankyrin 1 (TRPA1). The result is the transmission and perception of itch, but other mechanisms are still under discussion (2, 9–12). It is known that sensory nerve fibres express both TRPV1 and TRPA1 receptors and become directly stimulated by heat, and release neuropeptides that interact with other mediators.
Itch, transmitted along both histaminergic and non-histaminergic pathways, reflects the complex interplay between keratinocytes, immune cells, and cutaneous neurons (4, 9, 11, 13, 14). A clinically relevant consequence of the multifaceted itch mechanisms is the lack of a uniform anti-pruritic treatment. Various treatment strategies for chronic itch focus on 2 main areas. The first area targets peripheral itch mediators and receptors. This includes the use of steroids, antihistamines, calcineurin inhibitors, monoclonal antibodies, Janus kinase inhibitors, capsaicin, and ultraviolet (UV) phototherapy. The second area involves the nervous system, specifically signal transduction and inhibition. Treatments in this category encompass gamma-aminobutyric acid (GABA) agonists, opioid receptor modulators, nerve stimulation or inhibition, and various physical therapeutic approaches. Cannabinoids act both centrally and peripherally (7, 15–18). Former studies have shown that counterstimuli such as scratching, pinching, and noxious heat, the latter first used in itch management in the 1960s (19), are effective in inhibiting both histaminergic and non-histaminergic itch in vivo (20–22). The exact mechanism of action of noxious heat on itch remains to be elucidated, despite activation of inhibitory interneurons in the spinal cord, masking of itch signal by nociceptor activation, heat shock proteins, and TRPV1 desensitization having been implicated (23, 24).
The evidence on the use of noxious heat counterstimuli in itch management of atopic dermatitis (AD) patients is controversial and limited (25, 26). The current work aimed to show that short-term heat application (49°C) would lead to attenuation of experimentally induced itch and would suppress itch attacks of AD patients both in an experimental setting and in everyday life severity. Our primary hypothesis was that heat application (49°C) would reduce experimentally induced itch severity in AD patients, as witnessed by a visual analogue itch severity scale (VAS 0–10) compared with placebo treatment (room temperature). Areas under the curve (AUCs) were calculated as the outcome measure for this main objective (trapezoidal rule), relative to baseline. The secondary hypothesis was that heat application would result in a reduction in the measured time of itch sensation (in minutes) compared with placebo. In the following real-world study, we hypothesized that short-term, standardized heat application alleviates itch attacks of AD patients in everyday life witnessed by a reduction of itch intensity (VAS 0–10) over time.
MATERIAL AND METHODS
Study I
Design: To investigate the above hypothesis, we conducted a prospective randomized controlled trial from February 2022 to June 2022. Twelve subjects with mild to moderate active AD participated: heat (verum) and placebo (room temperature) were compared on randomized areas of the forearms with active eczema sites, applied after mechanically induced itching.
The study part I was divided into a visit (V1/on-site visit) and a phone call (V2/safety phone call). The on-site visit included study procedures, while V2 (the next day) was a telephone call to ask about adverse events or if the itching was still present.
Study subjects: Twelve female patients formerly diagnosed with AD according to Hanifin and Rajka criteria (27) and with active eczema lesions on both volar forearms were included. Table I gives the characteristics of the study subjects.
| Factor | Median | IQR |
| Age (years) | 38.00 | 10.0 |
| Height (cm) | 169.5 | 6.5 |
| Weight (kg) | 84.5 | 40.3 |
| Body mass index | 27.9 | 13.9 |
| IQR: interquartile range. | ||
All subjects had to meet all inclusion criteria: male or female volunteers aged 18–65 years, who provided verbal and written informed consent and were able and willing to comply with study procedures as per protocol; active eczema lesions on both volar forearms; TEWL values > 10 g/m² h (equivalent to at least mild epidermal barrier disruption), stratum corneum (SC) hydration values < 35 arbitrary units (AU) (equivalent to at least mild skin dryness); and no topical treatment with drug-containing or cosmetic externals on the volar forearms in the 48 h (drug-containing) or 24 h (cosmetic) before the start of the study.
Subjects meeting any of the following exclusion criteria were excluded from the study: subjects under 18 years of age; no verbal and written informed consent; use of medications that could affect skin physiological parameters (immunosuppressive treatment, calcineurin inhibitors, systemic and/or topical corticosteroids, antihistamines and/or medications with antihistamine effects); use of an investigational device or cosmetic within the last 30 days/5 half-lives of the drug in a clinical trial; institutionalization; pregnancy or breastfeeding.
Procedure. Subjects were given an acclimatization period of 20 min at a constant temperature of 20–21°C and air humidity of approximately 40%. Both interventions to be studied (heat and room temperature) were assigned to a skin area according to a randomization list. Each subject had a personalized template, which was not reused. Baseline assessment included monitoring of itch on a 10-point VAS, where 0 corresponds to no itch and 10 to the strongest imaginable itch (VAS 0–10) (in cm). The itch was measured every 60 s over 10 min by VAS by the individual patient. From these values, itch duration, maximum itch intensity, and AUC were determined over the measurement period for each test area (verum vs placebo).
Baseline values for SC hydration, skin redness (erythema), and transepidermal water loss (TEWL), as a measure of epidermal barrier function, were recorded. The following devices were used: Corneometer® CM 825 (Courage + Khazaka electronic GmbH, Cologne, Germany) to measure SC hydration; Mexameter® MX 18 (Courage + Khazaka electronic GmbH, Cologne), to measure skin erythema; Tewameter® TM 300 (Courage + Khazaka electronic GmbH, Cologne), to measure TEWL.
Itch induction was performed in a standardized manner by rubbing the skin with the rough surface of an unused wash sponge, simulating in a controlled manner friction/scratch-induced itch in AD (28, 29)). This model was chosen because AD patients are avoiding rough closing as an aggravating factor and because the mechanical scratching (e.g., with fingernails or scratching devices) aggravates AD symptoms. Painful sensations were not induced by this model. The rough sponge was moved 20 times (10 times forwards and 10 times backwards) with light pressure over the area of interest. The itch intensity was documented immediately after itch induction every minute for 10 min using VAS. Intervention (heat application/placebo) occurred 2 min after itch induction. After documenting itch intensity for 10 min, skin physiological parameters were determined again. A safety call was performed after 24 h.
The individual pain sensations evoked by the brief counterstimulus were not recorded in this study to not intervene with the assessment of the itch ratings.
Study II
Design: This prospective, real-life, interventional, uncontrolled, open-label study was conducted to disclose whether the use of heat-based, CE-approved device epiivo (https://epiivo.com/de/) (Fig. S1) could suppress itch attacks in patients with active mild-to-moderate AD.
Study subjects: Twelve patients (11 females and 1 male) diagnosed with AD according to the criteria of Hanifin and Rajka, and with active eczema lesions on both volar forearms, were included (Table II). The inclusion and exclusion criteria were identical to Study I.
| Factor | Median | IQR |
| Age (years) | 37.5 | 8.2 |
| Height (cm) | 172.5 | 13.8 |
| Weight (kg) | 84.0 | 38.0 |
| BMI | 26.1 | 10.3 |
| IQR: interquartile range. | ||
Procedure: Heat application with the epiivo device for itching in everyday life was performed with up to 20 applications over 7 days.
Baseline (itch within the last 24 h), at the beginning of the itch attack, immediately after application of the epiivo device, and 5 and 10 min after heat application assessment by VAS were performed. Study II was divided into 2 visits (on-site visits) for handing out and delivering the epiivo device, and at the end of the study for taking back the devices.
Epiivo medical device
The medical device “epiivo” (mibeTec GmbH, Brehna, Germany) is CE-marked according to the Medical Device Regulation (EU) 2017/745 (MDR), and was developed for thermo-therapeutic, topical symptomatic treatment of itching in acute and chronic pruritus. It is based on the application of a brief, concentrated thermal stimulus to a small, limited area of skin through a ceramic surface. The controlled heating from the heating module (approx. 47°C or 49°C, integrated microprocessor-controlled thermostat) is enabled with the internal connection between temperature and time control. In both studies, epiivo was used as a source of short-term noxious heat. In our study, we used the 49°C temperature for 5 s. Room temperature of the unheated device was chosen as a placebo. The investigation of medical devices typically involves sham use, which means the medical device is applied without activation of the function and the patient cannot detect this. In our case, as the heat is detectable this was not applicable. We therefore chose to explain to the patient that this is a medical device and that we wanted to test 2 different temperatures. One device was therefore applied at room temperature. In the results section this is called placebo.
Statistical analysis
The statistical analysis was performed with GraphPad Prism 6 (https://www.graphpad.com/). D’Agostino & Pearson’s omnibus test was used for normal distribution estimation. For pairwise comparison, in the case of normal distribution Student’s t-tests were employed; if non-normal distribution was seen, Wilcoxon tests were employed. The statistical significance level was set at p < 0.05.
Ethical aspects
Local authority approval by the Ethics Committee of the Charité – Universitätsmedizin Berlin (number EA1/315/2) was obtained. All study subjects provided verbal and written informed consent for study participation. The legal basis for the processing of personal data in scientific studies was the voluntary written consent according to the General Data Protection Regulation (GDPR) as well as the Declaration of Helsinki (Declaration of the World Medical Association on the Ethical Principles for Medical Research Involving Human Subjects) and the Guideline for Good Clinical Practice. Subjects who participated in the study were informed in detail about the processing of personal data and their right to withdraw consent. Consent to the processing of this data was considered a prerequisite for participation in the study.
Patients were recruited from the outpatient clinic at Charité – Universitätsmedizin Berlin, Institute of Allergology, following IRB-approved protocols for study participation.
RESULTS
Induced itch
Itch induction and heat application effectiveness, compared with placebo over 10 min, are shown in Fig. 1. Heat application at 49°C for 5 s initially increased itchiness until minute 3, followed by a steady decrease until minute 10. In contrast, the room temperature application (placebo) showed no significant reduction in itch severity. The time course of the itching sensation was assessed in terms of AUC, itch duration, and intensity, and is depicted in Fig. 2. Following heat application, the mean itch intensity was 161.0 AUC over the 10-min study period. In contrast, with placebo, the mean AUC values were 206.5 (Fig. 2A). The differences, however, were not statistically significant. The mean itch duration with heat application was 6.0 min, and with placebo, it was 8.7 min, showing a trend but not reaching statistical significance (p = 0.0742) (Fig. 2B). Similarly, the maximum itch intensity did not reach significance, with VAS values of 16.4 for heat and 20.2 for placebo (Fig. 2C). Subsequently, the effect of heat was tested on the individual level (Fig. 3): The individual itching curves of 12 test subjects were analysed. During heat intervention (Fig. 3A), itching initially increased but then decreased sharply. Itch reduction after mechanical stimulation was observed in only 5 of the 12 test subjects. No relevant itch induction beyond the basal itch could be provoked by the procedure in the eczema areas of 7 subjects. No significant trend in itch reduction was observed with the placebo (Fig. 3B). Skin physiology parameters (Fig. 4) under heat application (and placebo) were assessed in terms of TEWL (Fig. 4A) and SC hydration (Fig. 4B). TEWL showed a slight but not significant increase after the application of heat and placebo. SC hydration levels were low in active eczema lesions and showed only a discrete increase post-heat application. The erythema index, indicating skin redness, did not significantly increase after heat application (Fig. 4C).
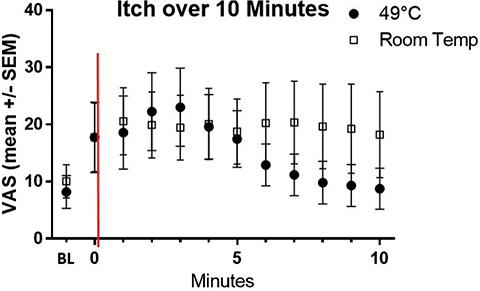
Fig. 1. Time course of itch induction and effect of heat application compared with room temperature (placebo) over 10 min. The x-axis represents experiment time in minutes, while the y-axis shows subjects’ average itch measured by VAS (mean +/– standard error of the mean (SEM)). The initial reading (–1) on the x-axis indicates baseline itch. The intensity of itch was recorded on a VAS from 0 (no itch) to 100 (maximum itch) (in mm). Itch is mechanically induced with a sponge and assessed every minute using VAS from minute 0. The intervention (heat 49°C 5 s (black-filled circles) or room temperature application (empty squares) with the epiivo device) is marked at time-point 0 (red line). Heat application initially increases itch until minute 3, followed by a steady decrease until minute 10. Conversely, room temperature application (placebo) shows no significant itch reduction.
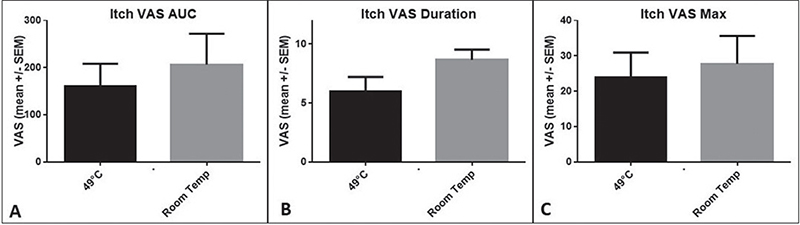
Fig. 2. Time course of itching sensation (AUC, itch duration, intensity). (A) Itch intensity (VAS) represented as AUC in the bar chart (mean +/– SEM). Following heat application (49°C, 5 s), the mean itch intensity (VAS) was 161.0 as AUC over the 10-min study period. In contrast, with the placebo (room temperature) application, the mean VAS value was 206.5 as AUC. However, the differences were not statistically significant (p = 0.3110; Wilcoxon test) due to the high standard error within the cohort. (B) Mean (+/– SEM) itch duration. With 49°C heat application the itch duration is 6.0 min, and with placebo application, it is 8.7 minu. In the direct comparison of heat application with placebo, the difference showed a significant trend (p = 0.0742; Wilcoxon test), missing the statistical significance level due to the high standard error. (C) Mean (+/– SEM) maximum itch intensity. The maximum itch intensity after itch induction with heat application (49°C) shows a VAS value of 16.4. With placebo application (room temperature), the maximum itch intensity shows VAS values of 20.2. The difference did not reach the significance level due to the high standard error.
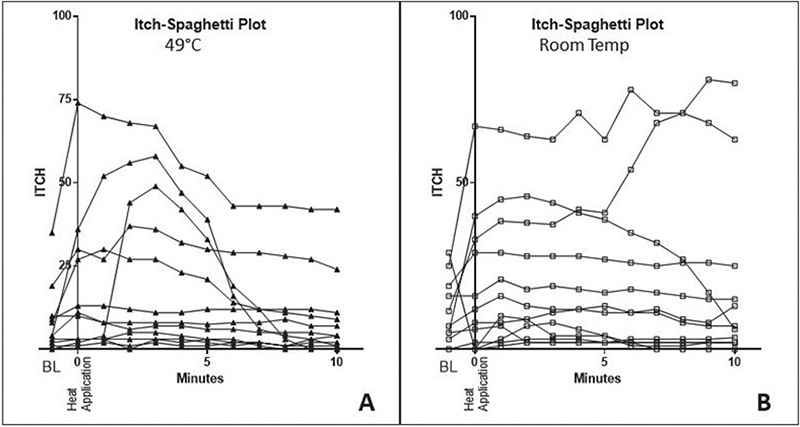
Fig. 3. Itch reduction on the individual level: heat effectivity is shown only in patients with induced itch augmentation. Spaghetti diagram: The x-axis represents the time course of the experiment in minutes for each volunteer, while the y-axis measures itch intensity using VAS. The individual itch curves of all 12 subjects are depicted. (A) During the intervention with heat (49°C), the itch initially increases, but then decreases sharply. However, an itch-modulating effect was observed in only 5 of 12 subjects. This is because no additional relevant itch could be mechanically induced in the eczema areas of 7 out of 12 subjects. (B) In contrast, no significant trend in the reduction of itch was observed when the placebo was administered.
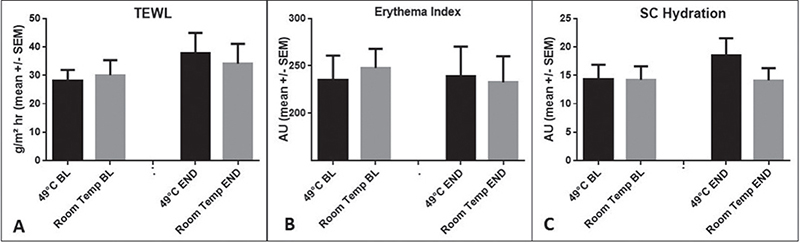
Fig. 4. Skin physiology parameters: no relevant changes induced by heat application. (A) TEWL as a parameter for the epidermal barrier function was measured with the Tewameter TM300. A characteristic of atopic dermatitis is disturbed epidermal barrier function, especially in active eczema lesions. In combination with the additional mechanical irritation to induce itch, a consistently increased TEWL was observed. There was no significant difference in the epidermal barrier function of the individual test areas before the application of 49°C heat and placebo. After the application of heat and placebo, there was a slight increase in the TEWL value, which showed only a trend without reaching a significance level (p = 0.0676) for both modalities with slightly (not significantly) higher values for the heat treatment. (B) SC-hydration was measured with the Corneometer CM 825. As heat was applied on active eczema lesions after previous mechanical itch induction, the relatively low values of approximately 15 AU, which corresponds to dry skin (normal skin is approximately 35–40 AU), were to be expected. The application of heat led only to a discrete increase in hydration of the stratum corneum, which was not significant. (C) The erythema index quantifies the redness of the skin and was measured as a parameter for the inflammatory reaction using the Mexameter MX16. The already basally increased erythema index due to the active eczema lesions was not significantly aggravated by the application of heat.
Real-life study
Part II was intended to assess itch reduction under real-life conditions during itch attacks with heat application (Fig. 5). The effect of short-term heat application on itching attacks in everyday life was investigated. Significant itch reduction was observed for all time points after heat application. The VAS values increased during the itching attack leading to the application of heat and significantly decreased immediately after heat application, with the decrease continuing over 5 and 10 min. We further assessed the efficacy of repeated heat applications over 7 days (Fig. 6). The itch intensity over the last 24 h and after repeated heat applications was examined. The results showed no significant change in itch intensity or loss of effectiveness of the heat treatment over time, even after up to 20 applications.
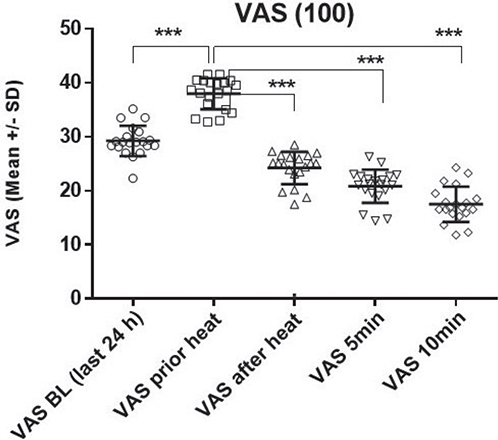
Fig. 5. Itch reduction in real-life heat application during itch attacks: immediate effect and follow-up over 10 min. Part II was conducted to investigate the effect of short-term heat application on itch attacks of atopic dermatitis patients in everyday life. The intensity of itch was recorded on a VAS from 0 (no itching) to 100 (maximum itching) (in mm). Itch intensity is summarized in connection with up to 20 heat applications within 24 h (first block) directly before and immediately after heat (49°C) application (induced by an itch attack), and over a 5-to-10 min follow-up period: the calculation by ANOVA revealed significant itch reduction (p < 0.0001) for all time points after heat application. The post-hoc pairwise comparison (including Bonferroni adjustment) showed a significant increase in the VAS itch values shortly before heat was applied (VAS before heat = itch attack) compared with the baseline of the last 24 h (VAS baseline [BL]) (p < 0.0001). This significant increase is attributable to the itching attack leading to the application of heat. Immediately after the application of heat (VAS after heat), there was a significant decrease in itch (p < 0.0001). In the follow-up period, there was a continued significant decrease in itch scores after 5 and 10 min (p < 0.0001).
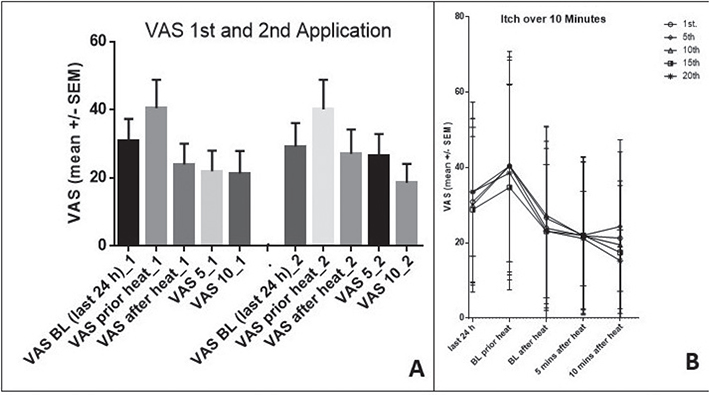
Fig. 6. Repeated applications: no loss of effectiveness. The itch intensity is shown for the last 24 h (BL) immediately before (itch attack), immediately after heat application (49°C for 5 s), and during the 5- and 10-min follow-up periods (left Panel A). Panel A depicts the first and second heat applications. Panel B (right) depicts the itch intensity after 1, 5, 10, 15, and 20 heat applications as VAS reported by the patients. The course does not change during the study period, and no loss of effectiveness could be observed.
DISCUSSION
Our study adds to the growing body of evidence that short-term heat application is an effective non-pharmacological intervention for reducing itch intensity in patients with AD (30–32). Previous studies have demonstrated a significant itch-reducing effect of the application of topical formulations in AD (33, 34). Preliminary studies have demonstrated itch reduction with heat application (20, 28, 35–38). In our study, a heat application of 49°C was used for 5 s. The same device without heat application (room temperature) served as a placebo in Study I. Although the main hypothesis of heat application leading to a significant reduction in itch severity compared with placebo treatment was not confirmed in the overall group, the trend observed toward itch duration reduction warrants attention. This suggests that while heat may not universally decrease itch severity, it could influence the duration of itch episodes.
Importantly, the individual patient analysis (spaghetti diagrams) revealed a subset of patients who responded positively to heat treatment. This individual variability in response to heat application suggests that patient-specific factors, possibly including AD severity, skin barrier integrity, and individual differences in nerve fibre density or sensitivity, play a role in the efficacy of heat as an anti-pruritic treatment (1, 10, 24, 31, 39, 40). The association between heat relief and relief during a warm shower in some patients further underscores the need for personalized approaches in managing AD symptoms.
In Study II, the significant and immediate reduction in itch intensity during real-life itch attacks following short-term heat application supports the efficacy of this intervention in a practical, everyday setting. The lack of habituation effect over the study period is encouraging for the long-term applicability of this method. Moreover, the observation that heat application did not lead to a significant increase in skin redness or exacerbate the pre-existing inflammatory state of the eczema lesions is critical, as it underscores the safety of this intervention.
Our findings raise intriguing questions regarding the mechanisms underlying itch relief via heat application. Heat may modulate neural pathways critical to itch perception, potentially through the activation of heat-sensitive receptors. This activation might interfere with pruritic signalling, possibly via mechanisms such as the activation of inhibitory interneurons in the spinal cord, nociceptors, heat shock proteins, and TRPV1 desensitization (23, 24). Moreover, the potential involvement of TRPA1 and TRPV1 receptors in this process highlights a complex interaction between thermal stimuli and itch modulation. These receptors, crucial for heat sensation and inflammatory responses, could underlie the itch-intensity reduction observed post heat application. The application of heat might alter typical itch signalling pathways through these receptors, suggesting a mechanism of action. Alternatively, heat could induce changes at the skin barrier level, affecting mediators or receptors involved in itch (41, 42).
Furthermore, the Gate Control Theory (43), which elucidates pain modulation, might also be applicable to the modulation of itch signals at the spinal level, supporting the notion that thermal activation of TRPA1 and TRPV1 could “gate” or inhibit itch transmission. This theory provides a neurophysiological basis for the observed therapeutic effects of heat on itch.
However, our analysis does not directly investigate the specific roles of TRPA1 and TRPV1, representing a limitation of the study. Additionally, the small sample size may limit the generalizability of our findings. Heat might also induce changes at the skin barrier level, influencing mediators or receptors implicated in itch signalling. Further research is imperative to clarify these mechanisms comprehensively, ascertain the roles of TRPA1 and TRPV1 specifically, and determine the optimal temperature and duration of heat application for maximal efficacy. Addressing the study’s limitations, future work should expand the sample size and explicitly explore the contributions of these receptors to thermal itch modulation.
The results from this study have important implications for the management of itch in AD, particularly in offering a safe, non-drug alternative for patients. However, it is essential to acknowledge the limitations of our study, including the small sample size and the specific nature of the heat application device used. Another limitation of our study is the chosen placebo (unheated device), as placebo response rates for medical devices are known to range from 30% up to 60% (44) We opted to compare with the non-heated device instead of completely untreated sites.
Future studies with larger cohorts and diverse patient populations are essential to validate these findings and explore the effectiveness of different heat application methods. Additionally, research into the long-term effects of repeated heat application and its impact on the quality of life in AD patients would be valuable.
In conclusion, our study provides promising evidence for the efficacy of short-term heat application as a non-pharmacological approach to managing itch in atopic dermatitis. The method’s safety, immediate effect, and lack of habituation over time suggest its potential as a practical intervention for AD patients. While individual responses vary, this approach offers a valuable addition to the therapeutic choice against chronic pruritus in AD, warranting further exploration and validation in large-scale studies.
ACKNOWLEDGEMENTS
This study was performed by Mandy Erdas at ECARF Institute, Berlin.
REFERENCES
- Cevikbas F, Lerner EA. Physiology and pathophysiology of itch. Physiol Rev 2020; 100: 945–982.
- Mahmoud O, Oladipo O, Mahmoud RH, Yosipovitch G. Itch: from the skin to the brain – peripheral and central neural sensitization in chronic itch. Front Mol Neurosci 2023; 16: 1272230.
- Misery L, Brenaut E, Pierre O, Le Garrec R, Gouin O, Lebonvallet N, et al. Chronic itch: emerging treatments following new research concepts. Br J Pharmacol 2021; 178: 4775–4791.
- Lipman ZM, Yap QV, Rosen J, Nattkemper L, Yosipovitch G. The association of chronic pruritus with patients’ quality of life: a cross-sectional study. J Am Acad Dermatol 2022; 86: 448–450.
- Stander S, Blome C, Breil B, Bruland P, Darsow U, Dugas M, et al. [Assessment of pruritus – current standards and implications for clinical practice: consensus paper of the Action Group Pruritus Parameter of the International Working Group on Pruritus Research (AGP)]. Hautarzt 2012; 63: 521–522, 524–531.
- Weisshaar E. Itch: A global problem? Front Med (Lausanne) 2021; 8: 665575.
- Weisshaar E, Szepietowski JC, Dalgard FJ, Garcovich S, Gieler U, Gimenez-Arnau AM, et al. European S2k guideline on chronic pruritus. Acta Derm Venereol 2019; 99: 469–506.
- Wohlrab J, Stintzing D, Schultz L, Jugelt K, Schroeder OH. Influence of Janus kinase inhibitors on the neuronal activity as a proof-of-concept model for itch. Skin Pharmacol Physiol 2022; 35: 94–101.
- Mahmoud O, Soares GB, Yosipovitch G. Transient receptor potential channels and itch. Int J Mol Sci 2022; 24.
- Guo CJ, Grabinski NS, Liu Q. Peripheral mechanisms of itch. J Invest Dermatol 2022; 142: 31–41.
- Misery L, Pierre O, Le Gall-Ianotto C, Lebonvallet N, Chernyshov PV, Le Garrec R, et al. Basic mechanisms of itch. J Allergy Clin Immunol 2023; 152: 11–23.
- Stefaniak AA, Agelopoulos K, Bednarska-Chabowska D, Mazur G, Stander S, Szepietowski JC. Small-fibre neuropathy in patients with type 2 diabetes mellitus and its relationship with diabetic itch: preliminary results. Acta Derm Venereol 2022; 102: adv00719.
- Lebonvallet N, Fluhr JW, Le Gall-Ianotto C, Leschiera R, Talagas M, Reux A, et al. A re-innervated in vitro skin model of non-histaminergic itch and skin neurogenic inflammation: PAR2-, TRPV1- and TRPA1-agonist induced functionality. Skin Health Dis 2021; 1: e66.
- Mochizuki H, Hernandez L, Yosipovitch G, Sadato N, Kakigi R. The Amygdala Network for processing itch in human brains. Acta Derm Venereol 2020; 100: adv00345.
- Roh YS, Choi J, Sutaria N, Kwatra SG. Itch: epidemiology, clinical presentation, and diagnostic workup. J Am Acad Dermatol 2022; 86: 1–14.
- Kupsa R, Gruber-Wackernagel A, Hofer A, Quehenberger F, Wolf P, Legat FJ. Narrowband-ultraviolet B vs broadband-ultraviolet B in treatment of chronic pruritus: a randomized, single-blinded, non-inferiority study. Acta Derm Venereol 2023; 103: adv9403.
- Badwy M, Baart SJ, Thio HB, Huygen F, de Vos CC. Electrical neurostimulation for the treatment of chronic pruritus: a systematic review. Exp Dermatol 2022; 31: 280–289.
- Nattkemper LA, Lipman ZM, Ingrasci G, Maldonado C Garces JC, Loayza E, et al. Neuroimmune mediators of pruritus in Hispanic scalp psoriatic itch. Acta Derm Venereol 2023; 103: adv4463.
- Hot water for itching. Med Lett Drugs Ther 1966; 8: 50–51.
- Riccio D, Andersen HH, Arendt-Nielsen L. Antipruritic effects of transient heat stimulation on histaminergic and nonhistaminergic itch. Br J Dermatol 2019; 181: 786–795.
- Lipshetz B, Giesler GJ, Jr. Effects of scratching and other counterstimuli on responses of trigeminothalamic tract neurons to itch-inducing stimuli in rats. J Neurophysiol 2016; 115: 520–529.
- Riccio D, Andersen HH, Arendt-Nielsen L. Mild skin heating evokes warmth hyperkinesis selectively for histaminergic and serotoninergic itch in humans. Acta Derm Venereol 2022; 102: adv00649.
- Sanchez-Moreno A, Guevara-Hernandez E, Contreras-Cervera R, Rangel-Yescas G, Ladron-de-Guevara E, Rosenbaum T, et al. Irreversible temperature gating in trpv1 sheds light on channel activation. Elife 2018; 7: e36372.
- Akiyama T, Carstens E. Neural processing of itch. Neuroscience 2013; 250: 697–714.
- Ishiuji Y, Coghill RC, Patel TS, Dawn A, Fountain J, Oshiro Y, et al. Repetitive scratching and noxious heat do not inhibit histamine-induced itch in atopic dermatitis. Br J Dermatol 2008; 158: 78–83.
- Fruhstorfer H, Hermanns M, Latzke L. The effects of thermal stimulation on clinical and experimental itch. Pain 1986; 24: 259–269.
- Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol 1980; Suppl 92: 44–47.
- Murota H, Katayama I. Exacerbating factors of itch in atopic dermatitis. Allergol Int 2017; 66: 8–13.
- Yosipovitch G, Duque MI, Fast K, Dawn AG, Coghill RC. Scratching and noxious heat stimuli inhibit itch in humans: a psychophysical study. Br J Dermatol 2007; 156: 629–634.
- Sesi J, Feldman SR. Comparative efficacy of systemic treatments for atopic dermatitis in adults. Expert Rev Clin Immunol 2023; 20: 313–320.
- Escalante A, Serra-Baldrich E. Pathogenic mechanisms underlying itch in atopic dermatitis: the emerging role of neuroimmune interactions. Eur J Dermatol 2023; 33: 343–349.
- Darlenski R, Kozyrskyj AL, Fluhr JW, Caraballo L. Association between barrier impairment and skin microbiota in atopic dermatitis from a global perspective: unmet needs and open questions. J Allergy Clin Immunol 2021; 148: 1387–1393.
- Silverberg JI, Pierce E, Feely M, Atwater AR, Schrader A, Jones EA, et al. Disease burden among patients with atopic dermatitis treated with systemic therapy for 4–12 months: results from the CorEvitas Atopic Dermatitis Registry. J Dermatolog Treat 2023; 34: 2246601.
- Rajkumar J, Chandan N, Lio P, Shi V. The skin barrier and moisturization: function, disruption, and mechanisms of repair. Skin Pharmacol Physiol 2023; 36: 174–185.
- Metz M, Elberskirch M, Reuter C, Liedtke L, Maurer M. Efficacy of concentrated heat for treatment of insect bites: a real-world study. Acta Derm Venereol 2023; 103: adv11592.
- Wohlrab J, Voss F, Muller C, Brenn LC. The use of local concentrated heat versus topical acyclovir for a herpes labialis outbreak: results of a pilot study under real life conditions. Clin Cosmet Investig Dermatol 2013; 6: 263–271.
- Muller C, Grossjohann B, Fischer L. The use of concentrated heat after insect bites/stings as an alternative to reduce swelling, pain, and pruritus: an open cohort-study at German beaches and bathing-lakes. Clin Cosmet Investig Dermatol 2011; 4: 191–196.
- Yosipovitch G, Fast K, Bernhard JD. Noxious heat and scratching decrease histamine-induced itch and skin blood flow. J Invest Dermatol 2005; 125: 1268–1272.
- Gustin J, Bohman L, Ogle J, Chaudhary T, Li L, Fadayel G, et al. Use of an emollient-containing diaper and pH-buffered wipe regimen restores skin pH and reduces residual enzymatic activity. Pediatr Dermatol 2020; 37: 626–631.
- Li J, Wang L, Yin S, Yu S, Zhou Y, Lin X, et al. Emerging trends and hotspots of the itch research: a bibliometric and visualized analysis. CNS Neurosci Ther 2023; 10.1111/cns.14514.
- Celebi Sozener Z, Treffeisen ER, Ozdel Ozturk B, Schneider LC. Global warming and implications for epithelial barrier disruption and respiratory and dermatologic allergic diseases. J Allergy Clin Immunol 2023; 152: 1033–1046.
- Lee CH, Hong CH, Liao WT, Yu HS. Differential immunological effects of infrared irradiation and its associated heat in vivo. J Photochem Photobiol B 2016; 155: 98–103.
- Melzack R, Wall PD. Pain mechanisms: a new theory. Science 1965; 150: 971–979.
- Kaptchuk TJ, Goldman P, Stone DA, Stason WB. Do medical devices have enhanced placebo effects? J Clin Epidemiol 2000; 53: 786–792.