SHORT COMMUNICATION
Successful Treatment of Mucous Membrane Pemphigoid with Dupilumab: A Case Report
Zhiyi WANG, Xiaojing LIU, Jing NI, Yushuo QI, Zhiqi SONG and Yongjun PIAO*
First Affiliated Hospital of Dalian Medical University, No.222. Zhongshan Road, Dalian, Liaoning, 116011, P.R. China. *E-mail: drpiao@163.com
Citation: Acta Derm Venereol 2024; 104: adv40162. DOI: https://doi.org/10.2340/actadv.v104.40162.
Copyright: © 2024 The Author(s). Published by MJS Publishing, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/).
Submitted: Feb 24, 2024. Accepted after revision: Aug 5, 2024. Published: Aug 26, 2024
INTRODUCTION
Mucous membrane pemphigoid (MMP) comprises a group of autoantibody-mediated subepidermal bullous diseases, characterized by a chronic course with predominant involvement of mucous membranes and a tendency to scarring. The oral mucosa (85% of patients) is the most common site, but conjunctiva (65%), nasal mucosa (20–40%), skin (20–35%), anus (20%), pharynx (20%), larynx (5–10%), and oesophagus (5–15%) can be affected (1, 2). Treatment of MMP can often be challenging. Currently, with the emergence of biologics and small-molecule drugs in dermatology, novel therapies such as rituximab and baricitinib are now being employed for the treatment of MMP. Nevertheless, it is crucial to acknowledge that the current range of therapeutic interventions available for MMP remains limited (3). In this case report, we present a case with MMP who exhibited a positive response to dupilumab. Dupilumab is a recombinant fully human IgG4 monoclonal antibody that binds specifically to the interleukin-4 receptor subunit alpha (IL-4Rα) shared by both human IL-4 and IL-13. This mechanism of action holds the potential to effectively inhibit the activity of both IL-4 and IL-13 (4).
CASE REPORT
A 36-year-old male patient with mouth ulceration for no obvious reason over 3 years ago repeatedly visited the external stomatology department, where histopathology and direct immunofluorescence examination indicated the presence of bullous pemphigoid, and the patient was prescribed methylprednisolone 32 mg (PO, QD), but with poor efficacy. Subsequently, the patient gradually experienced scattered blisters on the perineum and scalp, with erosions and bleeding in both nasal cavities. Additionally, there was redness and swelling of the eyelids, accompanied by conjunctival hyperaemia and blurred vision (Fig. 1). Cytokine assay showed interleukin 4 (IL-4) 9.55 pg/mL (ref ≤ 2.8). Serum-specific antibody test showed 5.15 RU/mL (ref ≤ 20) for desmoglein (Dsg) 1 antibody, 48.57 RU/mL (ref ≤ 20) for Dsg 3 antibody, 92.03 RU/mL (ref ≤ 20) for BP180 antibody, and 61.18 RU/mL (ref ≤ 20) for BP230 antibody. Dermatological examination showed negative Nikolsky’s sign. Pathological biopsy showed epidermal hyperplasia, intercellular oedema, subepidermal fissure formation, and superficial dermal infiltration of lymphocytes and small amounts of eosinophils (Fig. 2). Indirect immunofluorescence (ssIIF) using 1M NaCl salt-split skin test showed no obvious deposition of IgG, IgM, IgA, or C3.
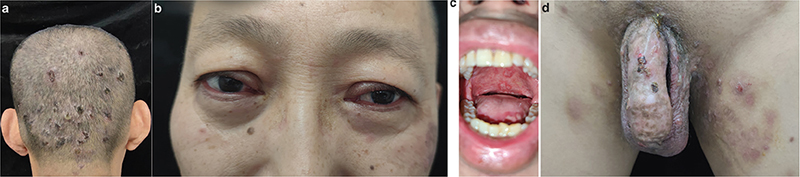
Fig. 1. Patient’s rash before treatment. A, D: Scattered blisters appeared on the perineum and scalp. B: Both eyelids became red and swollen, accompanied by conjunctival congestion. C: Scattered multiple oral ulcers.
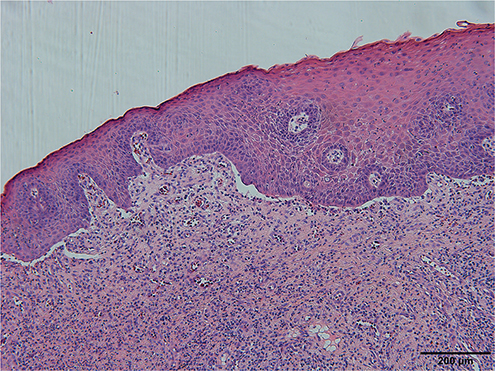
Fig. 2. Histopathological findings. Pathological tissue biopsy showed epidermal hyperplasia, intercellular oedema, subepidermal fissure formation, and infiltration of superficial dermal lymphocytes and a small amount of eosinophils.
The diagnosis of MMP was confirmed based on the patient’s clinical manifestations and relevant auxiliary examinations. An initial loading dose of 600 mg of dupilumab was administered subcutaneously, followed by 300 mg every 2 weeks, and supplemented with topical glucocorticoids and growth factors for the destruction of the peripheral skin and mucous membranes. After 3 months of regular treatment with dupilumab, the patient’s peripheral rash improved significantly, but the symptom of mouth ulceration and eyelid swelling did not improve significantly, so methylprednisolone 16 mg (PO, QD) was added. After 3 months of continued treatment, the symptoms of mouth ulceration and eyelid swelling were significantly relieved, therefore the dose of methylprednisolone was reduced to 5 mg QD. At present, the patient has been treated regularly with dupilumab for more than 10 months; all of the skin lesions and the ELISA values for BP180/230 have been significantly improved (Fig. 3). The most recent serum-specific antibody test showed 1.67 RU/mL for BP180 antibody and 1.54 RU/mL for BP230 antibody.
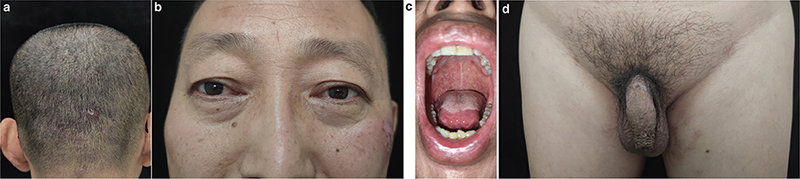
Fig. 3. Patient’s rash after 10 months of treatment with dupilumab. A–D: The scalp and perineal rash have improved significantly, the eyelid swelling has been completely relieved, and there is no obvious erosive surface in the oral cavity.
DISCUSSION
Treating MMP is challenging due to the presence of autoantibodies directed against components of the epidermal/epithelial basement membrane zone (BMZ). The primary target antigen of MMP is BP180 (collagen type XVII). In 10–20% of patients, reactivity to laminin 332 has been identified, and an association of laminin 332-specific antibodies with malignancies, predominantly solid tumours, has been demonstrated in approximately one-quarter of patients with MMP (5). In addition, collagen type VII is also recognized in less than 5% of patients, with a small amount of BP230 and integrin α6/β4 associated with the development of ocular lesions detected. Previously, mild MMP could be treated with potent topical corticosteroids, whereas moderate-to-severe conditions usually require systemic treatment with immunosuppressive drugs such as prednisone, azathioprine, mycophenolate mofetil, and cyclophosphamide. Biologics and small-molecule drugs (including rituximab, etanercept, infliximab, and baricitinib), and intravenous immunoglobulin (IVIG) can be selected for patients with refractory disease who fail to respond to the above treatments (6). These systemic therapies increase the risk of adverse effects, including infection and malignancy.
In this case report, cytokine assay showed elevated IL-4 in this patient, and there was almost no improvement despite treatment with high doses of oral methylprednisolone and topical steroids. Subsequently, the combination of dupilumab and low-dose glucocorticoid successfully improved the patient’s rash and pruritus. Although dupilumab is commonly used in the treatment of atopic dermatitis, asthma, and chronic rhinosinusitis with nasal polyps, its function as a monoclonal antibody against IL-4/IL-13 suggests its potential application in MMP, as both interleukins are indispensable for eosinophils and other inflammatory mediators in the pathogenesis of MMP (7).
Although reports of the use of dupilumab for the treatment of other bullous diseases (e.g., bullous pemphigoid) have been published, a search on PubMed using the terms “dupilumab” and “Mucous Membrane Pemphigoid” yielded only 1 case of successful treatment of Brunsting-Perry pemphigoid (BPP) with dupilumab (8). In this case, Although dupilumab alone can relieve the symptoms of MMP, it cannot be completely cured. The combination of small-dose glucocorticoids in this patient was significantly improved, suggesting that dupilumab is an effective steroid sparer, but additional studies are still needed to further demonstrate the potential benefits of dupilumab in patients with MMP (9).
ACKNOWLEDGEMENT
Consent: The authors obtained written consent from patients for their photographs and medical information to be published in print and online and with the understanding that this information may be publicly available. Patient consent forms were not provided to the journal but are retained by the authors.
REFERENCES
- Du G, Patzelt S, van Beek N, Schmidt E. Mucous membrane pemphigoid. Autoimmun Rev 2022; 21: 103036. https://doi.org/10.1016/j.autrev.2022.103036
- Rashid H, Lamberts A, Borradori L, Alberti-Violetti S, Barry RJ, Caproni M, et al. European guidelines (S3) on diagnosis and management of mucous membrane pemphigoid, initiated by the European Academy of Dermatology and Venereology - Part I. J Eur Acad Dermatol Venereol 2021; 35: 1750–1764. https://doi.org/10.1111/jdv.17397
- Olbrich H, Sadik CD, Schmidt E. Autoimmune blistering diseases: promising agents in clinical trials. Expert Opin Investig Drugs 2023; 32: 615–623. https://doi.org/10.1080/13543784.2023.2242778
- Russo R, Cozzani E, Gasparini G, Parodi A. Targeting interleukin 4 receptor α: a new approach to the treatment of cutaneous autoimmune bullous diseases? Dermatol Ther 2020; 33: e13190. https://doi.org/10.1111/dth.13190
- Rashid H, Meijer JM, Bolling MC, Diercks GFH, Pas HH, Horváth B. Insights into clinical and diagnostic findings as well as treatment responses in patients with mucous membrane pemphigoid: a retrospective cohort study. J Am Acad Dermatol 2022; 87: 48–55. https://doi.org/10.1016/j.jaad.2021.11.061
- Schmidt E, Rashid H, Marzano AV, Lamberts A, Di Zenzo G, Diercks GFH, et al. European Guidelines (S3) on diagnosis and management of mucous membrane pemphigoid, initiated by the European Academy of Dermatology and Venereology - Part II. J Eur Acad Dermatol Venereol 2021; 35: 1926–1948. https://doi.org/10.1111/jdv.17395
- Russo R, Capurro N, Cozzani E, Parodi A. Use of dupilumab in bullous pemphigoid: where are we now? J Clin Med 2022; 11: 3367. https://doi.org/10.3390/jcm11123367
- Raef HS, Elmariah SB. Successful treatment of Brunsting-Perry cicatricial pemphigoid with dupilumab. J Drugs Dermatol 2021; 20: 1113–1115.
- Maloney NJ, Tegtmeyer K, Zhao J, Worswick S. Dupilumab in dermatology: potential for uses beyond atopic dermatitis. J Drugs Dermatol 2019; 18: S1545961619P1053X.