SHORT COMMUNICATION
Single-system Langerhans Cell Histiocytosis with Skin Ulcers as the Initial Presentation
Xue-min WANG1, Ye-qiang LIU1, Bin LI1, Ming LI2, Yan PENG1 and Wen-cheng JIANG1*
1Shanghai Skin Disease Hospital of Tongji University, 1278 Baode Road, Jing’an District, Shanghai, 200443, China, and 2Children’s Hospital of Fudan University, Shanghai, China. *E-mail: drjiangwencheng@163.com
Citation: Acta Derm Venereol 2024; 104: adv40193. DOI: https://doi.org/10.2340/actadv.v104.40193.
Copyright: © 2024 The Author(s). Published by MJS Publishing, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/).
Submitted: Feb 27, 2024. Accepted after revision: Jun 17, 2024. Published: Aug 5, 2024
INTRODUCTION
Langerhans cell histiocytosis (LCH) is a rare disease of unknown aetiology caused by the clonal proliferation and infiltration of various organs by bone marrow-derived dendritic cells (DCs) in the bones, skin, lungs, pituitary gland, spleen, liver, central nervous system, and lymph nodes. Among them, cutaneous single-system Langerhans cell histiocytosis (SS-LCH) is relatively less frequent. Cutaneous manifestations often occur as an erythematous papular rash located in the groin or on the abdomen, chest, or back. However, cases of LCH with skin ulcers as the first presentation are extremely rare.
CASE DESCRIPTION
A 21-month-old female child presented with a 12-month history of right upper arm skin ulcers, after local vaccination with measles-mumps-rubella vaccine, hepatitis B vaccine, and group A and C meningococcal polysaccharide vaccine (Fig. 1A). The ulcers gradually developed from a local subcutaneous nodule. Adequate treatment with incision and drainage, dressing change (e.g., nanocrystalline silver dressing), and external use of human epidermal growth factor was ineffective.
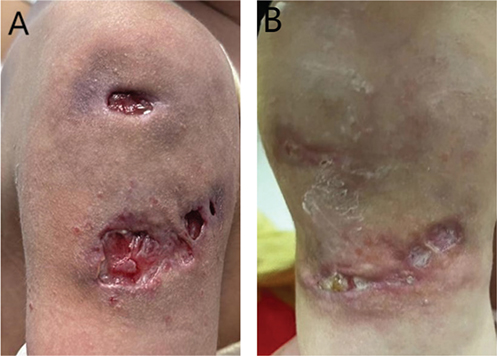
Fig. 1. Clinical photographs (right upper arm skin ulcers). (A) Pre-treatment. (B) Post-treatment at 4 months.
Histological examination exhibited an accumulation of large round to oval histiocyte-like cells with a complex nuclear contour that assumed a nuclear groove, expanding the papillary dermis at the epidermal–dermal interface (Fig. 2). The cells were strongly positive for CD1a and langerin/CD207 (Fig. S1), partially positive for BRAF, CD68, and LCA, mildly positive for S100 and CD117, but negative for CD30.
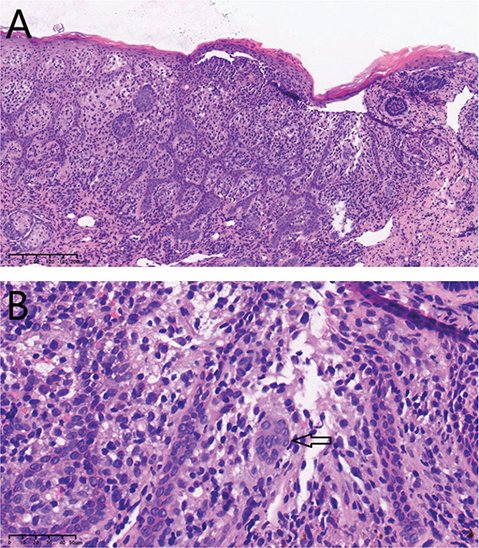
Fig. 2. Histological findings of current case. (A) Cutaneous LCH expanding the papillary dermis at the epidermal–dermal interface (haematoxylin and eosin (H&E), original magnification×10). (B) Higher power image of LCH in papillary dermis with nuclear grooves and mixed inflammatory infiltrate (H&E, original magnification×40), arrow points to LCH cells.
The results of whole-exome sequencing (this child and her parents) showed that no pathogenic genes were detected that could clearly explain the phenotype of the patient.
Laboratory examinations
Routine blood tests indicated a decrease in peripheral blood lymphocyte count and percentage. Lymphocyte subpopulation analysis showed a decreased T lymphocyte count and increased B lymphocyte count (Table SI). IgA and C4 were elevated, while IgG, IgM, and C3 were unremarkable (Table SII). Serum cytokine levels (IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-17, IL-10, IL-12p70, IFN-α, IFN-γ, and TNF-α) (Table SIII) and interferon-γ release assay (IGRA) did not reveal any abnormalities.
Imaging examinations
Posteroanterior cephalogram did not reveal any abnormalities. Chest X-ray revealed increased lung markings. Superficial organ ultrasonography revealed diffuse lesions that could be seen in the right thyroid area, with 2 intrathyroid nodules on the left side. There was mild enlargement of lymph nodes in the right neck.
Other examinations
The respiratory burst function of neutrophils was normal.
Treatment was commenced as follows: compound prednisone ointment (mainly composed of gentamicin, cod liver oil, tetracaine, and prednisone) on the ulcer edge and fusidic acid ointment on the ulcer surface. After 4 months of treatment, there was significant improvement (Fig. 1B).
DISCUSSION
Langerhans cell histiocytosis (LCH) is a clonal neoplasm of myeloid dendritic cells expressing a Langerhans cell phenotype (CD1a and CD207 expression). LCH can occur in any age group but is more common in children. The annual incidence rate in the paediatric population ranges from 2.6 to 8.9 cases per 1 million population (1), and in adults 1–2 cases per 1 million population (2).
Due to the rarity and heterogeneity of LCH, its pathogenesis has not been fully elucidated, and there has always been controversy over whether LCH is indicative of immune dysregulation or a neoplastic disorder. The associated Epstein–Barr virus infection with LCH and the detection of some virus DNA sequences in peripheral blood and tissues suggested that LCH is reactive (3, 4). LCH lesions include infiltrating pathological DCs (8%), macrophages, eosinophils, and lymphocytes enriched for regulatory T cells (5). Furthermore, the serum levels of various inflammatory cytokines and chemokines are high in LCH patients (6). However, the discovery of BRAF-V600E gene mutations and the mitogen-activated protein kinase (MAPK) pathway more supports the current clonal and neoplastic hypothesis. The mitogen-activated protein kinase (MAPK) pathway plays a central role in pathogenesis. A somatic activating mutation in a MAPK pathway gene has been identified in more than 85% of cases, with near universal phosphorylated ERK (p-ERK) upregulation and expression (7). The BRAF p.V600E mutation is the most prevalent (50–60%); MAP2K1 is the second most frequent (20–30%) (8). The BRAF V600E mutation renders the MAPK pathway constitutively active. Badalian-Very et al. (9) identified the BRAF V600E mutation in a remarkable 57% of LCH lesions, a finding that was subsequently verified in other series and attributed to the pathogenic LCH cell. The changes in the BRAF gene (BRAF V600E) that can support the diagnosis are relevant to the prognosis and management, and may expand treatment options to include targeted therapies (10).
LCH is classified into single-system (SS) and multisystem (MS) types based on the distribution of the affected lesions. The disease has different clinical courses, ranging from those that spontaneously resolve without treatment to those that rapidly progress and are fatal. Skin lesions are the second most common clinical manifestation of LCH, second only to bone involvement (5). Skin LCH is estimated to occur in 40–50% of paediatric LCH, but most are later associated with MS-LCH; SS-LCH of the skin is less frequent (11, 12).
The treatment varies depending on the disease type. Surgical excision of the lesion should be undertaken in very limited cases of SS-LCH with a solitary skin lesion (13). In skin-only cases, methotrexate, mercaptopurine, hydroxyurea, or thalidomide is also recommended. MS-LCH or SS-LCH with multifocal bone lesions is mainly treated via chemotherapy, including the standard 2-drug regimen with vinblastine and prednisone (5). In addition, the therapeutic armamentarium has broadened to include approaches such as targeted therapy and MAPK/ERK pathway inhibitors, which have achieved high response rates in children with refractory high-risk LCH (10). The patient in this case is an SS-LCH with skin involvement, so local treatment (topical use of glucocorticoids and antibiotics) can achieve a good curative effect. In addition, close follow-up is needed to enable timely intervention in cases of systemic involvement.
Furthermore, there is another case report of LCH related to vaccination (14). That child developed red-blue nodules on both buttocks after diphtheria, tetanus, and pertussis vaccination. Biopsy of the local lesions showed massive infiltration of Langerhans cells. He presented with extensive diffuse eczematous scaling lesions, pronounced on the scalp, trunk, and large body folds, where ulceration occurred. The patient later died of rapidly progressive MS disease with liver dysfunction. The report did not make it clear whether red-blue nodules after vaccination were the first presentation. However, it still suggests that localized skin rash after vaccination may not be accidental in LCH patients. We speculate as follows concerning the causes of cutaneous LCH induced by vaccination.
A small number of pathological Langerhans cells accumulate in the epidermal–dermal interface, but the damage to normal tissue is not significant, so there are no obvious clinical symptoms. Inflammation is a catalyser of tumour progression (15). Krall et al. (16) find that the systemic inflammatory response induced after surgery promotes the emergence of tumours whose growth was otherwise restricted by a tumour-specific T-cell response. Local injection of the vaccine as an antigen induces an excessive host immune response, leading to local inflammation. The continuous inflammation itself and the proliferation of tumour cells induced by inflammation destroy the surrounding normal tissue and induce the formation of ulcers, which are difficult to heal after adequate dressing change. This is consistent with the 2 current pathological hypotheses on LCH, namely inflammatory response and tumour. Therefore, a topical glucocorticoid anti-inflammatory is effective in this child. However, the child’s LCH is likely to recur again due to external antigenic stimulation.
In the past, there was a case of LCH with a refractory vulvar ulcer as the first manifestation, which was diagnosed definitively 3 years after onset (17). As such a manifestation can be a difficult diagnostic case, even for experienced dermatologists, we decided to publish this case report. A literature review of similar case reports will improve insight into the pathological mechanisms, diagnosis, and management.
REFERENCES
- Rodriguez-Galindo C, Allen CE. Langerhans cell histiocytosis. Blood 2020; 135: 1319–1331. https://doi.org/10.1182/blood.2019000934
- El-Arab KK, Luedke AI, Julian BQT, Ferrauiola J, Miller FR, Wang HTH. Langerhans cell histiocytosis in an adult: a discussion of epidemiology and treatment options. J Craniofac Surg 2020; 31: e70–e73. https://doi.org/10.1097/SCS.0000000000005925
- Sakata N, Toguchi N, Kimura M, Nakayama M, Kawa K, Takemura T. Development of Langerhans cell histiocytosis associated with chronic active Epstein-Barr virus infection. Pediatr Blood Cancer 2008; 50: 924–927. https://doi.org/10.1002/pbc.21249
- Murakami I, Matsushita M, Iwasaki T, Kuwamoto S, Kaot M, Horie Y, et al. Merkel cell polyomavirus DNA sequences in peripheral blood and tissues from patients with Langerhans cell histiocytosis. Hum Pathol 2014; 45: 119–126. https://doi.org/10.1016/j.humpath.2013.05.028
- Jezierska M, Stefanowicz J, Romanowicz G, Kosiak W, Lange M. Langerhans cell histiocytosis in children: a disease with many faces. Recent advances in pathogenesis, diagnostic examinations and treatment. Postepy Dermatol Alergol 2018; 35: 16–17. https://doi.org/10.5114/pdia.2017.67095
- Morimoto A, Oh Y, Nakamura S, Shioda Y, Hayase T, Imamura T, et al. Inflammatory serum cytokines and chemokines increase associated with the disease extent in pediatric Langerhans cell histiocytosis. Cytokine 2017; 97: 73–79. https://doi.org/10.1016/j.cyto.2017.05.026
- Allen CE, Merad M, McClain KL. Langerhans-cell histiocytosis. N Engl J Med 2018; 379: 856-868. https://doi.org/10.1056/NEJMra1607548
- Hayase T, Saito S, Shioda Y, Imamura T, Watanabe K, Ohki K, et al. Analysis of the BRAF and MAP2K1 mutations in patients with Langerhans cell histiocytosis in Japan. Int J Hematol 2020; 112: 560–567. https://doi.org/10.1007/s12185-020-02940-8
- Badalian-Very G, Vergilio JA, Degar BA, MacConaill LE, Brandner B, Calicchio ML, et al. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood 2010; 116: 1919–1923. https://doi.org/10.1182/blood-2010-04-279083
- Astigarraga I, García-Obregón S, Pérez-Martínez A, Gutiérrez-Carrasco I, Santa-María V, et al. Langerhans cell histiocytosis: advances in pathogenesis and clinical practice. An Pediatr 2022; 97: 130.e1–130.e7. https://doi.org/10.1016/j.anpede.2022.05.005
- Simko SJ, Garmezy B, Abhyankar H, Lupo PJ, Chakraborty R, Lim KPH, et al. Differentiating skin-limited and multisystem Langerhans cell histiocytosis. J Pediatr. 2014; 165: 990–996. https://doi.org/10.1016/j.jpeds.2014.07.063
- Fraitag S, Emile JF. Cutaneous histiocytoses in children. Histopathology 2022; 80: 196–215. https://doi.org/10.1111/his.14569
- Kobayashi M, Tojo A. Langerhans cell histiocytosis in adults: advances in pathophysiology and treatment. Cancer Sci 2018; 109: 3707–3713. https://doi.org/10.1111/cas.13817
- Morren M-A, Vanden Broecke KV, Vangeebergen L, Sillevis-Smitt JH, Van Den Berghe P, Hauben E, et al. Diverse cutaneous presentations of Langerhans cell histiocytosis in children: a retrospective cohort study. Pediatr Blood Cancer 2016; 63: 486–492. https://doi.org/10.1002/pbc.25834
- de Visser KE, Joyce JA. The evolving tumor microenvironment: from cancer initiation to metastatic outgrowth. Cancer Cell 2023; 41: 374–403. https://doi.org/10.1016/j.ccell.2023.02.016
- Krall JA, Reinhardt F, Mercury OA, Pattabiraman DR, Brooks MW, Dougan M, et al. The systemic response to surgery triggers the outgrowth of distant immune-controlled tumors in mouse models of dormancy. Sci Transl Med 2018; 10: eaan3464. https://doi.org/10.1126/scitranslmed.aan3464
- Khoummane N, Guimeya C, Lipombi D, Gielen F. Vulvar Langerhans cell histiocytosis: a case report. Pan Afr Med J 2014; 18: 119. https://doi.org/10.11604/pamj.2014.18.119.3204