SHORT COMMUNICATION
Long-term Efficacy of Dupilumab in Papulo-erythroderma of Ofuji
Clélia VANHAECKE1, Laurence GUSDORF1 and Manuelle VIGUIER1,2
1Department of Dermatology Hopital Robert-Debré Avenue du General Koenig, FR-51092 Reims CEDEX, and 2University of Reims Champagne-Ardenne (URCA), EA7509 IRMAIC, Reims, France. E-mail: cvanhaecke@chu-reims.fr
Citation: Acta Derm Venereol 2024; 104: adv40220. DOI https://doi.org/10.2340/actadv.v104.40220.
Copyright: 2024 © The Author(s). Published by MJS Publishing, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/).
Submitted: Feb 29, 2024; Accepted: May 15, 2024; Published: Jun 7, 2024
INTRODUCTION
Papulo-erythroderma of Ofuji (PEO) is a rare condition, characterized by red pruriginous papules sparing the skin folds (deck-chair sign) (1). It occurs classically in elderly men and is associated with blood eosinophilia and lymphocytopenia (1, 2). The aetiology is unknown, while association with visceral malignancies, atopic dermatitis, or psoriasis has been reported (2). Some authors suggested that PEO could be a precursor of cutaneous T-cell/epidermotropic lymphoma (3). Recent reports have suggested that T helper type 2 (Th2) cells could be important in the pathogenesis of PEO (4). Dupilumab is an interleukin (IL)-4 receptor α-antagonist that inhibits Th2-type immune reaction. We report herein the rapid and durable efficacy of dupilumab in an elderly woman with PEO.
CASE REPORT
A woman in her 80s presented to our department with a pruriginous eruption, occurring concomitantly with a septic arthritis of the shoulder. Exanthema started before the initiation of 6 weeks’ treatment with cefazoline. Her medical history included hypertension and Alzheimer’s dementia and she received aspirin, nicardipine, and oxazepam. Because of dementia, personal or family atopic histories were unknown. Physical examination revealed papulous erythema with predominance on the trunk with deck-chair sign (Fig. 1). The face and mucous membranes were not involved. Blood test revealed eosinophilia (1200 G/L) and elevated serum IgE (4,270 UI/mL, N < 114). Skin biopsy showed spongiosis with polymorphous interstitial dermis infiltrate with eosinophils with negative direct immunofluorescence. Diagnosis of PEO was confirmed.
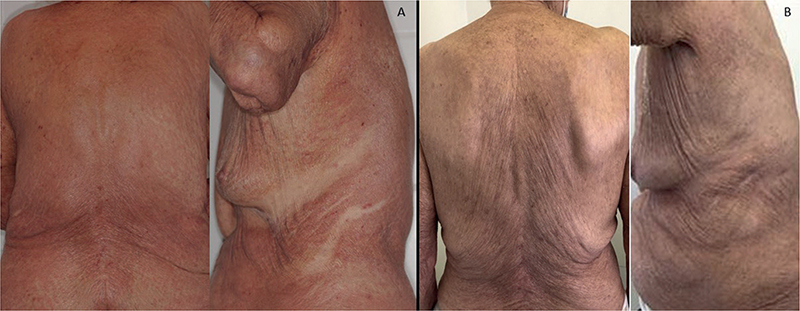
Fig. 1. Clinical evolution of papulo-erythroderma of Ofuji following dupilumab treatment. (A): Erythroderma with deck-chair sign before dupilumab. (B): Complete clinical remission after 3 months of dupilumab.
Topical corticosteroids (dipropionate betamethasone 0.05%) were initially efficient, but relapse occurred after stopping treatment. Because of recent septic arthritis, we were not inclined to introduce systemic corticosteroids. Dupilumab (600 mg at initial dosing and then 300 mg every 2 weeks) was therefore started. Cutaneous lesions and pruritus were dramatically improved during the first month, and complete remission was achieved at 3 months. Blood eosinophil count substantially decreased following treatment to 660 G/L and serum IgE to 2430 UI/mL. After 12 months, because of complete remission, dupilumab injections were spaced every 3 weeks for 1 year without recurrence, then spaced every 4 weeks for 6 months and finally stopped (total duration of treatment: 30 months). With a current follow-up of 6 months after the last dupilumab injection, no relapse has occurred. No adverse effect related to dupilumab was noted.
DISCUSSION
PEO is a rare disease, classically occurring in elderly patients, and is often refractory to conventional therapies. The average duration of PEO is unknown and the follow-up reported in the literature is very variable, from a few months to several years (5). A recent review reported that only a proportion of patients with PEO responded well to treatment with oral corticosteroids, PUVA, oral retinoids, or a combination of PUVA with either topical corticosteroids or oral retinoids (5). The best response was observed with oral retinoids (complete remission in 7 patients out of 8 treated) (5). When achieved, the time to complete response varied from 25 days with oral retinoids or oral corticosteroids to more than 100 days for PUVA. Some authors also reported efficacy of methotrexate in PEO (6). On the other hand, safety in this population can be an issue as topical or systemic corticosteroids can induce severe side effects (diabetes, high blood pressure, osteoporosis, dermatoporosis), and methotrexate is frequently contraindicated because of anaemia or kidney failure in elderly patients. Finally, PUVA therapy is becoming less and less available in France.
The inhibition of the Th2 pathway in PEO therefore represents an attractive treatment approach. The safety of dupilumab was recently also confirmed in elderly atopic patients, without new side effects reported (7). Two articles previously suggested the efficacy of dupilumab in PEO. Teraki et al. (8) reported 2 elderly men with PEO refractory to systemic and topical steroids with rapid complete remission with dupilumab at 4 months. After 4 months, dupilumab injections were spaced every 4 weeks in both patients. PEO remission was persistent 10 months after spacing in 1 patient. For the other patient, dupilumab injections were later spaced every 6 weeks without PEO relapse 9 months later. No cessation of dupilumab was reported. Komatsu-Fujii et al. (9) reported a 65-year-old man with PEO refractory to topical and systemic corticosteroids who achieved complete remission with dupilumab at 14 weeks without relapse 4 weeks after dupilumab cessation. We report, with our case, the longest follow-up including 30 months of dupilumab treatment and 6 months’ follow-up after dupilumab cessation, without PEO relapse and without adverse events.
Our case confirms the rapid and long-term efficacy of dupilumab for PEO with good tolerance. The use of dupilumab may be an option for the treatment of PEO by inhibiting Th2 type immune reaction.
REFERENCES
- Ofuji S, Furukawa F, Miyachi Y, Ohno S. Papuloerythroderma. Dermatologica 1984; 169: 125–130.
- Torchia D, Miteva M, Hu S, Cohen C, Romanelli P. Papuloerythroderma 2009: two new cases and systematic review of the worldwide literature 25 years after its identification by Ofuji et al. Dermatol Basel Switz 2010; 220: 311–320.
- Shah M, Reid WA, Layton AM. Cutaneous T-cell lymphoma presenting as papuloerythroderma: a case and review of the literature. Clin Exp Dermatol 1995; 20: 161–163.
- Teraki Y, Inoue Y. Skin-homing Th2/Th22 cells in papuloerythroderma of Ofuji. Dermatol Basel Switz 2014; 228: 326–331.
- Mufti A, Lytvyn Y, Abduelmula A, Kim P, Sachdeva M, Yeung J. Treatment outcomes in patients with papuloerythroderma of Ofuji: a systematic review. JAAD Int 2021; 3: 18–22.
- Balestri R, Magnano M, Rech G, Zorzi MG, Girardelli CR. Long-term use of methotrexate in papuloerythroderma of Ofuji. Dermatol Ther 2020; 33: e13219.
- P Patruno C, Fabbrocini G, Longo G, Argenziano G, Ferrucci SM, Stingeni L, et al. Effectiveness and safety of long-term dupilumab treatment in elderly patients with atopic dermatitis: a multicenter real-life observational study. Am J Clin Dermatol 2021; 22: 581–586.
- Teraki Y, Taguchi R, Takamura S, Fukuda T. Use of dupilumab in the treatment of papuloerythroderma of Ofuji. JAMA Dermatol 2019; 155: 979–980.
- Komatsu-Fujii T, Nonoyama S, Ogawa M, Fukumoto T, Tanabe H. Rapid effects of dupilumab treatment on papuloerythroderma of Ofuji. J Eur Acad Dermatol Venereol 2020; 34: e739–e741.