ORIGINAL REPORT
Acral Melanoma Incidence and Survival Trends in 1990–2020: A Nationwide, Population-based Study
Teo HELKKULA1,2, Gustav CHRISTENSEN1,2, Rasmus MIKIVER3, Åsa INGVAR1,2, Karolin ISAKSSON1,4 and Kari NIELSEN1,2,5
1Department of Clinical Sciences Lund, Lund University Skin Cancer Research Group, Lund University, Lund, 2Department of Dermatology and Venereology, Skåne University Hospital, Lund, 3Clinical and Experimental Medicine, Linköping University, Linköping, 4Department of Surgery, Kristianstad Hospital, Kristianstad, and 5Department of Dermatology, Helsingborg Hospital, Helsingborg, Sweden
Acral melanoma is a clinical subtype of melanoma with high mortality, on which research is limited in scope. This study aimed to assess incidence trends and melanoma-specific survival rates for acral melanoma in the Swedish population from 1990 to 2020.This cross-sectional study included patients with an acral melanoma diagnosis from 1990 to 2020 from the nationwide, population-based Swedish Melanoma Registry. Analyses on acral melanoma melanoma-specific survival rates were adjusted for age, sex, histopathological subtype, and tumour thickness. Clinicopathological features and melanoma-specific survival rates were compared between diagnostic periods: 1990–1999, 2000–2009, and 2010–2020, respectively. Changes in standardized incidence rates in 1996–2020 were evaluated separately for males and females. In total, 1,000 acral melanomas in 999 patients were included in the study. No significant yearly change in standardized incidence rates for either males or females was observed, even though the absolute number of cases increased. Factors such as male sex, age ≥ 70 years, and Breslow thickness > 1.0 were independently linked to lower melanoma-specific survival. The 5-year melanoma-specific survival across the studied period ranged from 75.8% to 77.9% for females, and from 62.4% to 71.7% for males.
Key words: incidence; melanoma; survival rate.
SIGNIFICANCE
This 1990 to 2020 study on acral melanoma, melanoma that arises on non-hair-bearing skin on the palms, soles, and nail beds, found that standardized occurrence rate and mortality rates have remained steady. This contrasts with the sharp rise in overall melanoma occurrence. Females and persons younger than 70 years had a lower risk of dying from acral melanoma. Still, mortality in acral melanoma is noticeably higher than in overall melanoma and extra attention needs to be given to enhance diagnostics in males and patients aged 70 or more, as their mortality rates in acral melanoma are especially concerning.
Citation: Acta Derm Venereol 2024; 104: adv40242. DOI https://doi.org/10.2340/actadv.v104.40242.
Copyright: © 2024 The Author(s). Published by MJS Publishing, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/).
Submitted: Mar 4, 2024; Accepted after revision: Jun 24, 2024; Published: Aug 14, 2024
Corr: Teo Helkkula, Lasarettsgatan 15, 22185, Lund, Sweden. E-mail: teo.helkkula@med.lu.se
Competing interests and funding: KI has received a speaker honorarium from Pierre Fabre. KN has received speaker honoraria from Galderma Sweden, LEO Pharma, Novartis Sweden, and UCB and has served on 1 advisory board for MSD. GC has received speaker honoraria from LEO Pharma and UCB. ÅI has received speaker honoraria and advisory fees from Galderma Sweden, Perrigo Sweden, MSD, and Biofrontera Sweden. The companies did not influence the study’s design, data collection, analysis, interpretation, or reporting. All other authors declare no conflicts of interest.
KN: Grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF agreement, the Gyllenstiernska Krapperup Foundation, the S.R. Gorthon Foundation, the Inga and John Hain Foundation, and the Welander-Finsen Foundation (Hudfonden). KI: Grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF agreement. ÅI: Swedish Cancer Society (Cancerfonden), Hudfonden. The sponsors did not influence study design, data collection, data analysis, manuscript preparation, or publication decisions.
INTRODUCTION
Cutaneous melanoma, a rapidly increasing cancer in fair-skinned populations, not least in Sweden, is a severe concern (1, 2). Acral melanoma (AM) is a subtype of melanoma originating from the skin on the palms, soles, and nail beds (3). AMs are often hypopigmented, and therefore frequently mistaken for other conditions, which together with the fact that plantar AMs are often difficult for the patient to discover, results in significant diagnostic delays (4, 5). As a result, AMs usually exhibit greater Breslow depths and mortality rates than other cutaneous melanomas (6).
Interestingly, the incidence of AM does not vary much across different skin pigmentation levels, remaining fairly constant between 0.04 and 0.25 per 100,000 person-years (7). Even though AM accounts for about 3% of all melanomas in fair-skinned populations, its proportion is significantly higher among populations with darker skin (8). According to the World Health Organization classification of melanoma, AM development is, unlike the majority of cutaneous melanomas, not consistently associated with cumulative solar damage (CSD) (3). The cause of AM is still unknown, but mechanical stress has been considered a possible contributing factor (9–11).
It is important to note that AM refers to melanomas defined by their acral location, whereas acral lentiginous melanoma (ALM) denotes a histopathological subtype in those areas. The terms AM and ALM are often misused and misunderstood, further compounded by previous studies’ lack of specification regarding anatomical sites. The limited epidemiological data on AM and these contributing factors make interpreting and comparing past AM studies challenging (8). Filling the knowledge gaps in AM survival and incidence will improve our understanding of this rare subtype of melanoma, enhancing our ability to diagnose and treat the disease effectively.
This study used nationwide, population-based data from the Swedish Melanoma Registry (SweMR) to assess incidence trends and melanoma-specific survival (MSS) rates for AM in the Swedish population from 1990 to 2020.
MATERIALS AND METHODS
Data were retrieved from the SweMR, a comprehensive nationwide population-based quality registry. Since 1990, clinical and histopathological data for patients with invasive cutaneous melanoma have been voluntarily recorded in the SweMR (12). From 1996, registration in the SweMR became requested for all healthcare regions in Sweden, resulting in an impressive coverage rate of 99%, compared with the mandatory National Cancer Registry. Within the SweMR, each melanoma diagnosis is intricately linked to the patients’ unique personal identity number, ensuring secure synchronization with the National Cause of Death Registry (13).
This study included all individuals in Sweden diagnosed with invasive melanoma on an acral site (palm, sole, or subungual) from 1990 to 2020. We observed these individuals’ MSS from the diagnosis date until either their demise from melanoma, the first instance of death from other causes, diagnosis of a second melanoma, or the end of follow-up on 31 December 2020.
The SweMR database provided clinicopathological information, which included the patient’s sex (according to the population registry), age (categorised as < 40, 40–59, 60–69, 70–79, and ≥ 80 years), Breslow thickness (divided into 0.1–1.0, 1.1–2.0, 2.1–3.0, 3.1–4.0, and > 4.0 mm), histopathological subtypes; acral lentiginous melanoma (ALM), superficial spreading melanoma (SSM), nodular melanoma (NM), lentigo maligna melanoma (LMM), other types of melanomas and unspecified melanomas, and T-stage (segmented as T1, T1a–b, T2, T2a–b, T3, T3a–b, T4, T4a–b). The T-staging followed the American Joint Committee on Cancer’s 8th edition staging for invasive melanoma (14). As of 1990, SweMR started registering the tumour site. However, until 2009, the term “acral site” was used without specifying the lower or upper extremities. Beginning in 2009, melanomas on acral sites were identified as either “palm or subungual finger” or “sole or subungual toe”.
Statistical analysis
AM cases were reported as absolute numbers and incidence rates per 100,000 person-years, age-standardized to the year 2000 population in Sweden and supplementary to the World Health Organization (WHO) standard population 2000. To assess changes in incidence rates starting in 1996, because SweMR registration was not yet fully nationwide during the initial years, an annual percentage change (APC) calculation was performed using joinpoint regression, with an alpha level of 0.05 (15, 16). The joinpoint analysis did not reveal any statistically significant fluctuating changes in incidence, leading to the adoption of a 0 joinpoint model.
For the analysis of time-dependent changes in clinicopathological features and MSS, the study period spanning from 1990 to 2020 was divided into 3 segments: period 1 (1990–2000), period 2 (2001–2010), and period 3 (2011–2020). This subdivision assessed alterations in clinicopathological features and MSS over time and facilitated comparisons with previous reports. Additionally, the choice of these periods took into account the introduction of new therapies during the latest study period.
Multinomial logistic regression, including age, sex, histopathologic subtype, and Breslow thickness, was employed to examine differences in tumour characteristics over time, with period 1 (1990–2000) as the reference period. The results are presented as odds ratios (ORs) and 95% confidence intervals (CIs).
Kaplan–Meier curves were plotted for overall MSS and separated by sex for different periods. To identify risk factors for melanoma-specific mortality (MSM), we conducted a multivariable Cox proportional hazard regression, incorporating variables such as sex, age, Breslow thickness, histopathological subtype, and period of diagnosis. Stratification was done for age and Breslow thickness to examine whether changes in prognosis were restricted to specific groups. Hazard ratios (HRs) are presented with 95% CIs and p-values from two-tailed tests. A significance level of < 0.05 was used. Statistical analyses were conducted using R Statistical Software (v4.0.3; RCore Team 2020; R Foundation for Statistical Computing, Vienna, Austria) and Joinpoint Trend Analysis Software (v.4.6.0.0; the National Cancer Institute; https://surveillance.cancer.gov/joinpoint/).
RESULTS
The absolute numbers and percentages of age, sex, and clinicopathological data in relation to study periods are listed in Table I. Between 1990 and 2020, 1,000 AMs were recorded in 999 people. An increase in AM counts was observed from period 1 to period 3. In period 1, there were 289 recorded AMs, compared with 308 in period 2 and 403 in period 3. The median age at diagnosis was 69, 71, and 72 years for each period. AMs were more prevalent in females in all periods, with the highest disparity occurring in period 2, where females and males accounted for 61.4% and 38.6% of cases respectively. This distribution lessened slightly in period 3, where it was 57.8% females and 42.2% males.
| Diagnostic period | 1990–2000 | 2001–2010 | 2011–2020 | Total |
| Total number of AMs | 289 | 308 | 403 | 1000 |
| Sex, n (%): | ||||
| Male | 132 (45.7) | 119 (38.6) | 170 (42.2) | 421 (42.1) |
| Female | 157 (54.3) | 189 (61.4) | 233 (57.8) | 579 (57.9) |
| Age, n (%) | ||||
| < 40 years | 22 (7.61) | 23 (7.47) | 19 (4.7) | 64 (6.4) |
| 40–59 years | 63 (21.8) | 62 (20.1) | 74 (18.4) | 199 (19.9) |
| 60–69 years | 67 (23.2) | 60 (19.5) | 76 (18.9) | 203 (20.3) |
| 70–79 years | 78 (27.0) | 79 (25.6) | 126 (31.3) | 283 (28.3) |
| ≥ 80 years | 59 (20.4) | 84 (27.3) | 108 (26.8) | 251 (25.1) |
| Median age [IQR] | 69 [57;77] | 71 [58;81] | 72 [60;80] | 71 [59;80] |
| Histopathological subtype, n (%): | ||||
| ALM | 104 (36.0) | 131 (42.5) | 183 (45.4) | 418 (41.8) |
| LMM | 5 (1.7) | 4 (1.3) | 6 (1.5) | 15 (1.5) |
| SSM | 71 (24.6) | 60 (19.5) | 98 (24.3) | 229 (22.9) |
| NM | 63 (21.8) | 50 (16.2) | 42 (10.4) | 155 (15.5) |
| Other | 42 (14.5) | 53 (17.2) | 55 (13.6) | 150 (15.0) |
| Missing | 4 (1.38) | 10 (3.3) | 19 (4.7) | 33 (3.3) |
| Site*, n (%): | ||||
| Palm or subungual finger | 0 (0.0) | 14 (4.6) | 95 (23.6) | 109 (10.9) |
| Sole or subungual toe | 0 (0.0) | 56 (18.2) | 308 (76.4) | 403 (40.3) |
| Acral site, unspecified | 289 (100.0) | 238 (77.3) | 0 (0.0) | 527 (52.7) |
| Breslow thickness (mm), n (%): | ||||
| 0.1–1.0 | 64 (22.1) | 53 (17.2) | 86 (21.3) | 203 (20.3) |
| 1.1–2.0 | 55 (19.0) | 63 (20.5) | 77 (19.1) | 195 (19.5) |
| 2.1–4.0 | 67 (23.2) | 73 (23.7) | 100 (24.8) | 240 (24.0) |
| > 4.0 | 76 (26.3) | 86 (27.9) | 108 (26.8) | 270 (27.0) |
| Missing | 27 (9.3) | 33 (10.7) | 32 (7.9) | 92 (9.2) |
| T category, n (%): | ||||
| T1 | 64 (22.1) | 53 (21.2) | 86 (20.3) | 203 (20.3) |
| T1a | 25 (8.7) | 29 (9.4) | 50 (12.4) | 104 (10.4) |
| T1b | 31 (10.7) | 19 (6.2) | 34 (8.4) | 84 (8.4) |
| T2 | 55 (19.0) | 63 (20.5) | 77 (19.1) | 195 (19.5) |
| T2a | 31 (10.7) | 41 (13.3) | 55 (13.6) | 127 (12.7) |
| T2b | 16 (5.5) | 17 (5.5) | 22 (5.5) | 55 (5.5) |
| T3 | 67 (23.2) | 73 (23.7) | 100 (24.8) | 240 (24.0) |
| T3a | 22 (7.6) | 22 (7.1) | 37 (9.2) | 81 (8.1) |
| T3b | 34 (11.8) | 48 (15.6) | 60 (14.9) | 142 (14.2) |
| T4 | 76 (26.3) | 86 (27.9) | 108 (26.8) | 270 (27.0) |
| T4a | 9 (3.1) | 17 (5.5) | 23 (5.7) | 49 (4.9) |
| T4b | 57 (19.7) | 68 (22.1) | 79 (19.6) | 204 (20.4) |
| Missing | 27 (9.3) | 33 (10.7) | 32 (7.9) | 92 (9.2) |
| *Information only available after year 2009. | ||||
| Values for T1, T2, T3, and T4 include a, b and unknown ulceration status. AM: acral melanoma; IQR: interquartile range; ALM: acral lentiginous melanoma; LMM: lentigo maligna melanoma; SSM: superficial spreading melanoma; NM: nodular melanoma. | ||||
Over time, the ratio of AMs decreased in younger and middle-aged groups (< 40, 40–59, and 60–69 years) and increased in older groups (70–79, and ≥ 80 years). Because of different registration standards pre- and post-2009, the exact AM locations were documented only in period 3. Here, 23.6% of AMs were found on the palm or subungual finger and 76.4% on the sole or subungual toe. The most common histological subtype was ALM (about 40%), followed by SSM. Some cases were classified as LMM even though they were on an acral site. Generally, LMM on these sites should be reported as ALM. However, the SweMR registration included a few LMM cases, which were accounted for separately. The LMM proportion remained consistent throughout all periods. Alternatively, ALM increased (36.0%, 42.5%, and 45.4%), while NM decreased from 21.8% to 16.2% to 10.4% over the 3 periods. No substantial variation was noted in different tumour thickness categories (Breslow thickness). Approximately half of the AMs had a tumour thickness of > 2.0 mm, consistent across all periods. Similar numbers were noted for tumour thicknesses of 2.1–4.0 and > 4.0 mm.
Table II illustrates changes in tumour attributes over time, referencing the first period. The ORs for AM per age group were notably higher in the third period for age groups 70–79 (2.16, 95% CI 1.04–4.48) and ≥ 80 years (2.30, 95% CI 1.09–4.86) compared with the reference age group (< 40 years). In the second period, females presented a higher OR of AM (1.46, 95% CI 1.02–2.07) than males, a trend that did not continue into the third period. Referring to the first period, the second and third periods showed significantly lower ORs for NM (second period: 0.57, 95% CI 0.35–0.92 and third period: 0.34, 95% CI 0.21–0.55) compared with the ALM in the first period. No significant differences were identified in ORs for other histopathological subtypes or the distribution of tumour thickness across the study periods.
| Variable | 2001–2010 OR (95% CI) | 2011–2020 OR (95% CI) |
| Intercept | 0.77 (0.37–1.61) | 0.88 (0.42–1.84) |
| Age | ||
| < 40 years | 1 (ref) | 1 (ref) |
| 40–59 years | 1.02 (0.49–2.10) | 1.30 (0.62–2.73) |
| 60–69 years | 0.90 (0.43–1.86) | 1.50 (0.72–3.14) |
| 70–79 years | 1.00 (0.49–2.06) | 2.16 (1.04–4.48)* |
| ≥ 80 years | 1.31 (0.63–2.73) | 2.30 (1.09–4.86)* |
| Sex | ||
| Male | 1 (ref) | 1 (ref) |
| Female | 1.46 (1.02–2.07)* | 1.22 (0.88–1.70) |
| Histopathologic subtype | ||
| Acral lentiginous melanoma | 1 (ref) | 1 (ref) |
| Lentigo maligna melanoma | 0.71 (0.17–2.99) | 0.64 (0.17–2.36) |
| Superficial spreading melanoma | 0.65 (0.42–1.02) | 0.74 (0.49–1.11) |
| Nodular melanoma | 0.57 (0.35–0.92)* | 0.34 (0.21–0.55)* |
| Other | 0.90 (0.52–1.55) | 0.69 (0.41–1.17) |
| Breslow thickness (mm) | ||
| 0.1–1.0 | 1 (ref) | 1 (ref) |
| 1.1–2.0 | 1.37 (0.81–2.33) | 1.08 (0.66–1.76) |
| 2.1–4.0 | 1.38 (0.82–2.31) | 1.12 (0.70–1.80) |
| > 4.0 | 1.52 (0.90–2.57) | 1.23 (0.75–1.99) |
| Period 1990–2000: 1 (reference), OR: odds ratio; CI: confidence interval. | ||
| *p-value < 0.05. | ||
The age-standardized incidence rates per 100,000 person-years for the 3 periods are shown in Table SI for 1996–2000, 2001–2010, and 2011–2020. The rates were 0.37, 0.33, and 0.38, respectively, for the total population. For males, the rates were 0.23, 0.16, and 0.19, and for females, 0.22, 0.23, and 0.24, respectively. For comparison, incidence rates standardized by the 2000 WHO standard population are also shown (Table SI). Incidence trends from 1996–2020, presented as APC, can be seen in Fig. 1. The APC was 0.94 (95% CI -0.61–2.52) for females and -0.33 (95% CI -1.86–1.22) for males with 0 joinpoints, which mark the inflexion points of the fitted lines. However, these APCs were not statistically significant.
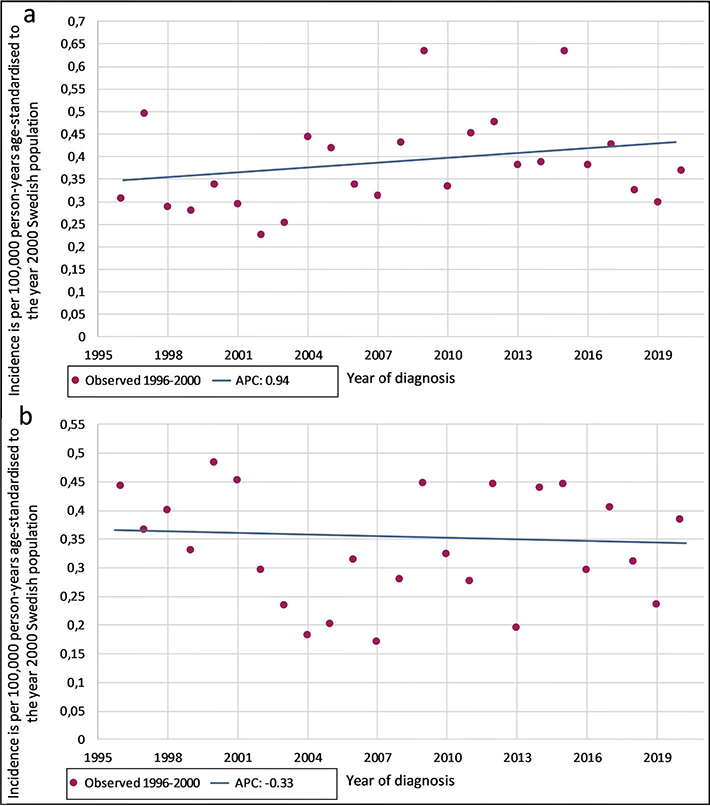
Fig. 1. Annual percentage change (APC) in incidence of acral melanomas, using joinpoint analysis with 0 joinpoints, for (a) females and (b) males. Incidence is per 100,000 person-years and age-standardized to the year 2000 Swedish population.
MSS is divided by study periods and displayed in Fig. 2. Neither the 5-year MSS curves for the total population of the study nor the curves for males and females indicated any significant difference between the periods. Table SII presents the corresponding 5-year MSS (95% CIs) as percentages.
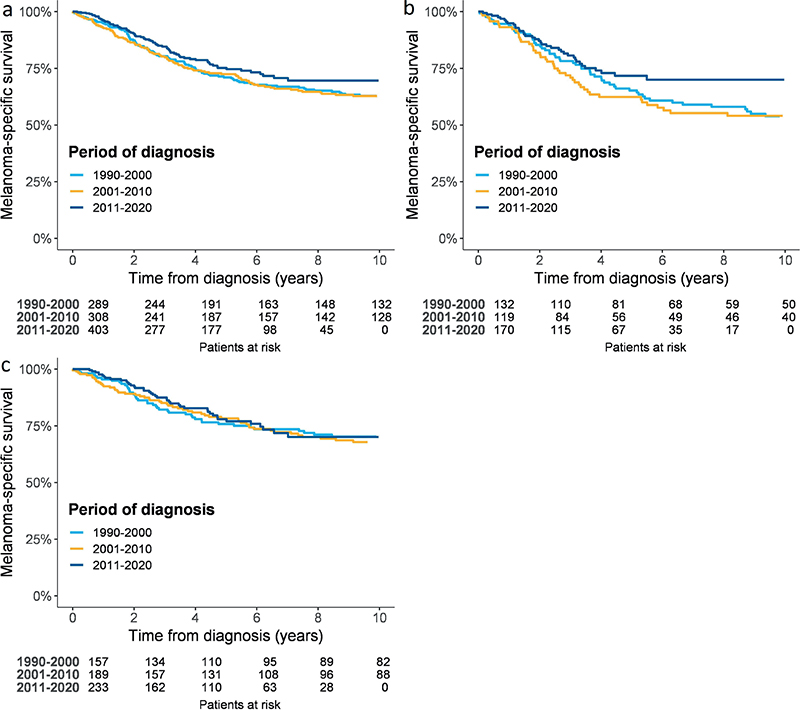
Fig. 2. Kaplan–Meier curves display 5-year melanoma-specific survival 1990–2020 for (a) the total study population, (b) males, and (c) females.
The multivariable Cox regression analysis demonstrated a nearly 30% lower risk for MSM among females than males (HR 0.73, 95% CI 0.57–0.95), as indicated in Table III. The risk of MSM significantly rose with advancing age, especially within the 70–79 and 80 and above age groups. It also increased significantly with higher Breslow thickness. Finally, the histopathological subtypes of SSM and NM, compared with ALM, displayed an association with an increased risk of MSM. This association was nearly significant, with HR 1.40, CI 0.99–1.97, and HR 1.35, CI 0.97–1.87, respectively.
| Multivariable Cox | HR (95% CI) | p-value |
| Sex | ||
| Male | 1 (ref) | |
| Female | 0.73 (0.57–0.95) | 0.020* |
| Age | ||
| < 40 years | 1 (ref) | |
| 40–59 years | 2.10 (0.94–4.69) | 0.070 |
| 60–69 years | 2.00 (0.90–4.42) | 0.087 |
| 70–79 years | 2.63 (1.20–5.75) | 0.016* |
| ≥ 80 years | 3.57 (1.60–7.92) | 0.002* |
| Breslow thickness, mm | ||
| 0.1–1.0 | 1 (ref) | |
| 1.1–2.0 | 2.67 (1.44–4.97) | 0.002* |
| 2.1–4.0 | 5.62 (3.16–10.02) | < 0.001* |
| > 4.0 | 9.17 (5.15–16.35) | < 0.001* |
| Histopathologic subtype | ||
| Acral lentiginous melanoma | 1 (ref) | |
| Lentigo maligna melanoma | 0.94 (0.23–3.85) | 0.932 |
| Superficial spreading melanoma | 1.40 (0.99–1.97) | 0.057 |
| Nodular melanoma | 1.35 (0.97–1.87) | 0.076 |
| Other | 1.14 (0.76–1.70) | 0.523 |
| Year of diagnosis | ||
| 1990–2000 | 1 (ref) | |
| 2001–2010 | 0.95 (0.70–1.27) | 0.713 |
| 2011–2020 | 0.74 (0.53–1.02) | 0.067 |
| HR: hazard ratio. | ||
| *p-value < 0.05. | ||
DISCUSSION
Data from the SweMR, a Sweden-wide, population-based study conducted from 1990–2020 found no significant shifts in the age-standardized incidence rates (expressed as APC) or MSS. The frequency of AM cases in individuals over 70 years old showed an increasing trend, which aligns with overall melanoma statistics in Sweden (2). This is understandably a by-product of an ageing population. Factors associated with an increased MSM were age over 70 and a Breslow thickness over 1.0 mm. Despite most AM patients being female, they had a lower MSM risk compared with males.
The comprehensive registration in SweMR allows for meticulous evaluation of time patterns. Unlike the swiftly escalating general incidence of cutaneous melanoma, Sweden has seen no rise in AM incidence during the researched periods (17). International information on AM incidence is sparse, complicating direct comparisons. Nonetheless, ALM incidence trends can serve as a surrogate for AM incidence, and our results align with past studies on ALM in the United States and East-Central Europe (18–20).
The incidence of AM differs from other types of melanomas, likely due to its largely unknown causes. However, 1 proposed risk factor is mechanical stress, given that AM often appears on weight-bearing areas of the feet and is most commonly found on the thumb and great toenails (9–11). Supporting this theory, Jung et al. (9) reported that subungual AMs were more frequently found on fingers than toes, and those on the fingers mainly appeared on the right side, reinforcing the role of mechanical stress. By extension, it is reasonable to hypothesize a correlation between socioeconomic status (SES), lifestyle, and AM. Higher SES, often associated with better footwear and a less physically demanding lifestyle, should ideally reduce mechanical stress and subsequently reduce the incidence of AM. Additionally, given that prosperity and living standards have generally improved over time, the incidence of AM might be expected to decline, that is, if mechanical stress truly is one of the most significant risk factors for AM. However, a US study contradicts this theory, finding that ALM was more prevalent among higher SES non-Hispanic whites and Asian or Pacific Islanders, while the reverse was true for Hispanics and non-Hispanic blacks (21). These findings do not definitively support the proposed connection between mechanical stress and AM, as measured by SES and ALM. Unfortunately, the current data are insufficient to draw any robust conclusions.
The AM’s stable incidence contrasts sharply with the rising incidence of melanomas in other skin areas. This discrepancy may be attributed to the increased UV exposure among fair-skinned groups (22, 23). Another possible reason for the overall increase in melanoma incidence could be the earlier diagnosis of cutaneous melanomas, particularly SSM, due to the advancement in diagnostic methods such as dermatoscopy (24).
Public awareness of melanomas’ typical signs, mainly those of SSM, has increased due to frequent information campaigns, which may influence SSM’s incidence and overdiagnosis (25). In Sweden, this trend is evident for non-acral melanomas but not for AM, corroborating a study of ALM in East-Central Europe (20). Consequently, it appears either that early stages of AM are stabilizing or that symptoms of early-stage AM do not prompt individuals to seek medical attention (2).
Unfortunately, there has been no significant change in the diagnostic delay or the poor prognosis of AM in the Swedish population. More than half of the AMs registered in the SweMR were tumours greater than 2.0 mm in thickness, compared with slightly over a fifth of all melanomas (2). This could be due to their often hypopigmented appearance and misdiagnosis as benign conditions, resulting in diagnostic delay (26). Additionally, particularly among the elderly, early symptoms in the lower extremities can be missed by patients, their families or healthcare professionals at elderly or nursing homes.
These findings underline the importance of public and medical professional education concerning the warning signs of AM. This is affirmed by our study, where the MSM risk remained nearly constant over time. However, our study found a nearly significant decrease in MSM for period 3, potentially because of effective metastatic disease therapies available during the study period. It is, however, important to note that AMs exhibit a considerably lower frequency of BRAF mutations than cutaneous melanoma overall, reducing BRAF inhibitor utility, and few studies have evaluated the effect of immune checkpoint inhibitors on AM (27). Another possible explanation for the tendency of decreasing mortality is the lower ratio of NMs, a melanoma subtype with high mortality risk (28).
Our study also found female sex to be an independent favourable prognostic factor, aligning with previous studies on melanoma (29–32). Similar to our findings, previous research on both AM (33) and ALM (34, 35) indicated a predominance of female cases, and male sex as an independent adverse prognostic factor. However, Vikstrom et al. (2) found no significant gender disparities in MSS among melanoma patients in Sweden. Interestingly, a US study showed no difference in ALM incidence between the sexes (18). These findings suggest the need for further studies to investigate potential biological or behavioural sex differences, particularly in relation to AMs, in Sweden.
Research suggests that AM is a deadlier subtype of melanoma compared to those found in non-acral locations (6, 36). However, most studies primarily report survival outcomes for ALM, rather than AM (19, 37). In our study, we found that the 5-year MSS rates for males (62.4–71.7%) and females (75.8–77.9%) with AM were lower than the overall melanoma survival rates in Sweden during the same period, which ranged from 88.9–93.0% as noted by Vikström et al. (2). It it essential to note that direct comparison would require adjustment for factors such as as tumour thickness.
On an international scale, the ALM subtype has been reported to have a comparable survival to acral SSM when adjusted for stage (36, 38), but worse prognosis when not adjusting for stage or Breslow thickness (37). Our study suggests that ALM shows tendencies of better survival rates than SSM and NM in acral locations. These findings align with a Swedish study comparing MSS for SMM, NM, and ALM (39). Both studies predominantly examined fair-skinned individuals, which compose the majority of the Swedish population (40). In the SweMR, only a few LMM cases emerged in acral sites. The few instances of LMM in this study were likely misclassified as LMM instead of ALM, given that LMM located on acral sites should be classified as ALM. As ALM and LMM are classified as melanomas of non-CSD/high CSD, differences in mutational profiles of the tumours might be found, but such diagnostic details were not available in this study. Regardless, given the low number and consistency over time, we do not foresee a significant misclassification bias in our study results.
Strength and limitations
The principal strength of this study lies in its population-based approach, and completeness of the collection because it comes from a national registry, leveraging detailed data gathered from the SweMR over a significant length of time. These comprehensive data allow for the comparison and validation of results, thereby enhancing our understanding of AM. These findings should be applicable to other primarily fair-skinned populations with similar exposures. However, the study is potentially limited by incomplete registrations of certain variables during the initial period. Nevertheless, given that these missing values only make up a minor fraction of our data, they are unlikely to affect the results significantly. Although we utilised nationwide data observed over an extended period, another limitation is that AMs are rare tumours. A larger case sample would further reinforce our findings. Lastly, due to the lack of precise tumour location details during the entire study, we were unable to compare tumours on the upper versus lower extremities throughout all periods.
Conclusion
The incidence and mortality rates of AM in Sweden have remained stable over time. Notably, the proportion of AM is higher among females than males, yet the prognosis remains less favourable for males. Individuals aged 70 years and older face the highest risk of developing and dying from AM, and this age group has seen an increase in the prevalence of these tumours over time. There is a pressing need for enhanced public and healthcare professional awareness and education regarding warning signs, aiming to improve diagnostic efforts and reduce mortality rates associated with AM.
ACKNOWLEDGEMENTS
The authors would like to extend their appreciation to the individuals in Sweden who generously contributed their data and the dedicated clinicians and national cancer centres responsible for collecting data for vital Swedish national registries, including the SweMR and the Swedish Melanoma Study Group.
IRB approval status: The study was approved by the Swedish Ethical Review Authority (Dnr 2020-03870).
REFERENCES
- Arnold M, Singh D, Laversanne M, Vignat J, Vaccarella S, Meheus F, et al. Global burden of cutaneous melanoma in 2020 and projections to 2040. JAMA Dermatol 2022; 158: 495–503. https://doi.org/10.1001/jamadermatol.2022.0160
- Vikström S, Mikiver R, Lapins J, Nielsen K, Vassilaki I, Lyth J, et al. Increasing melanoma incidence and survival trend shifts with improved melanoma-specific survival between 1990 and 2020 in Sweden. Br J Dermatol 2023; 189: 702–709. https://doi.org/10.1093/bjd/ljad244
- Elder DE, Bastian BC, Cree IA, Massi D, Scolyer RA. The 2018 World Health Organization classification of cutaneous, mucosal, and uveal melanoma: detailed analysis of 9 distinct subtypes defined by their evolutionary pathway. Arch Pathol Lab Med 2020; 144: 500–522. https://doi.org/10.5858/arpa.2019-0561-RA
- Tan KB, Moncrieff M, Thompson JF, McCarthy SW, Shaw HM, Quinn MJ, et al. Subungual melanoma: a study of 124 cases highlighting features of early lesions, potential pitfalls in diagnosis, and guidelines for histologic reporting. Am J Surg Pathol 2007; 31: 1902–1912. https://doi.org/10.1097/PAS.0b013e318073c600
- Soon SL, Solomon AR Jr, Papadopoulos D, Murray DR, McAlpine B, Washington CV. Acral lentiginous melanoma mimicking benign disease: the Emory experience. J Am Acad Dermatol 2003; 48: 183–188. https://doi.org/10.1067/mjd.2003.63
- Lv J, Dai B, Kong Y, Shen X, Kong J. Acral melanoma in Chinese: a clinicopathological and prognostic study of 142 cases. Sci Rep 2016; 6: 31432. https://doi.org/10.1038/srep31432
- Durbec F, Martin L, Derancourt C, Grange F. Melanoma of the hand and foot: epidemiological, prognostic and genetic features. A systematic review. Br J Dermatol 2012; 166: 727–739. https://doi.org/10.1111/j.1365-2133.2011.10772.x
- Bernardes SS, Ferreira I, Elder DE, Nobre AB, Martínez-Said H, Adams DJ, et al. More than just acral melanoma: the controversies of defining the disease. J Pathol Clin Res 2021; 7: 531–541. https://doi.org/10.1002/cjp2.233
- Jung HJ, Kweon SS, Lee JB, Lee SC, Yun SJ. A clinicopathologic analysis of 177 acral melanomas in Koreans: relevance of spreading pattern and physical stress. JAMA Dermatol 2013; 149: 1281–1288. https://doi.org/10.1001/jamadermatol.2013.5853
- Sheen YS, Liao YH, Lin MH, Chen JS, Liau JY, Tseng YJ, et al. A clinicopathological analysis of 153 acral melanomas and the relevance of mechanical stress. Sci Rep 2017; 7: 5564. https://doi.org/10.1038/s41598-017-05809-9
- Lee JH, Choi YD, Hwang JH, Shin MH, Yun SJ. Frequency of trauma, physical stress, and occupation in acral melanoma: analysis of 313 acral melanoma patients in Korea. Ann Dermatol 2021; 33: 228–236. https://doi.org/10.5021/ad.2021.33.3.228
- The Swedish Melanoma Registry – SweMR National annual report for cutaneous melanoma. Available from: https://cancercentrum.se/globalassets/om-rcc/sydost/pdf/nationell-kvalitetsregisterrapport-hudmelanom-1990-2021.pdf.
- Lyth J, Mikiver R, Nielsen K, Ingvar C, Olofsson Bagge R, Isaksson K. Population-based prognostic instrument (SweMR 2.0) for melanoma-specific survival: an ideal tool for individualised treatment decisions for Swedish patients. Eur J Surg Oncol 2023; 49: 106974. https://doi.org/10.1016/j.ejso.2023.06.026
- Gershenwald JE SR, Hess KR. AJCC cancer staging manual. 8th ed. New York: Springer Cham, 2017.
- National Cancer Institute – Joinpoint Trend Analysis Software. Available from: https://surveillance.cancer.gov/joinpoint/
- Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 2000; 19: 335–351. https://doi.org/10.1002/(SICI)1097-0258(20000215)19:3<335::AID-SIM336>3.0.CO;2-Z
- Eriksson H, Nielsen K, Vassilaki I, Lapins J, Mikiver R, Lyth J, et al. Trend shifts in age-specific incidence for in situ and invasive cutaneous melanoma in Sweden. Cancers (Basel) 2021; 13: 2838. https://doi.org/10.3390/cancers13112838
- Huang K, Fan J, Misra S. Acral lentiginous melanoma: incidence and survival in the United States, 2006–2015, an analysis of the SEER Registry. J Surg Res 2020; 251: 329–339. https://doi.org/10.1016/j.jss.2020.02.010
- Bradford PT, Goldstein AM, McMaster ML, Tucker MA. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986–2005. Arch Dermatol 2009; 145: 427–434. https://doi.org/10.1001/archdermatol.2008.609
- Csányi I, Houshmand N, Szűcs M, Ócsai H, Kemény L, Oláh J, et al. Acral lentiginous melanoma: a single-centre retrospective review of four decades in East-Central Europe. J Eur Acad Dermatol Venereol 2020; 34: 2004–2010. https://doi.org/10.1111/jdv.16227
- Raval NS, Hodges WT, Ugwu-Dike PO, Godoy F, Ansstas G, Cornelius LA, et al. Racial and socioeconomic differences in acral lentiginous melanoma outcomes: a surveillance, epidemiology, and end results analysis. J Am Acad Dermatol 2022; 87: 866–867. https://doi.org/10.1016/j.jaad.2021.11.023
- Arnold M, de Vries E, Whiteman DC, Jemal A, Bray F, Parkin DM, et al. Global burden of cutaneous melanoma attributable to ultraviolet radiation in 2012. Int J Cancer 2018; 143: 1305–1314. https://doi.org/10.1002/ijc.31527
- Erdei E, Torres SM. A new understanding in the epidemiology of melanoma. Expert Rev Anticancer Ther 2010; 10: 1811–1823. https://doi.org/10.1586/era.10.170
- Kurtansky NR, Dusza SW, Halpern AC, Hartman RI, Geller AC, Marghoob AA, et al. An epidemiologic analysis of melanoma overdiagnosis in the United States, 1975–2017. J Invest Dermatol 2022; 142: 1804–1811.e1806. https://doi.org/10.1016/j.jid.2021.12.003
- Matsumoto M, Wack S, Weinstock MA, Geller A, Wang H, Solano FX, et al. Five-year outcomes of a melanoma screening initiative in a large health care system. JAMA Dermatol 2022; 158: 504–512. https://doi.org/10.1001/jamadermatol.2022.0253
- Boriani F, O’Leary F, Tohill M, Orlando A. Acral lentiginous melanoma: misdiagnosis, referral delay and 5 years specific survival according to site. Eur Rev Med Pharmacol Sci 2014; 18: 1990–1996.
- Mao L, Qi Z, Zhang L, Guo J, Si L. Immunotherapy in acral and mucosal melanoma: current status and future directions. Front Immunol 2021; 12: 680407. https://doi.org/10.3389/fimmu.2021.680407
- Elshanbary AA, Zaazouee MS, Abdelmonem M, Mohammed YA, Abdel-Aziz W. Risk factors for cardiovascular mortality and melanoma-specific mortality among patients with melanoma: a SEER based study. Eur J Cancer Prev 2022; 31: 293–300. https://doi.org/10.1097/CEJ.0000000000000690
- Morgese F, Sampaolesi C, Torniai M, Conti A, Ranallo N, Giacchetti A, et al. Gender differences and outcomes in melanoma patients. Oncol Ther 2020; 8: 103–114. https://doi.org/10.1007/s40487-020-00109-1
- Nader Marta G, Munhoz RR, Teixeira MP, Waldvogel BC, Pires de Camargo V, Feher O, et al. Trends in melanoma mortality in Brazil: a registry-based study. JCO Glob Oncol 2020; 6: 1766–1771. https://doi.org/10.1200/GO.20.00426
- Briatico G, Mancuso P, Argenziano G, Longo C, Mangone L, Moscarella E, et al. Trends in cutaneous melanoma mortality in Italy from 1982 to 2016. Int J Dermatol 2022; 61: 1237–1244. https://doi.org/10.1111/ijd.16173
- de Vries E, Nijsten TE, Visser O, Bastiaannet E, van Hattem S, Janssen-Heijnen ML, et al. Superior survival of females among 10,538 Dutch melanoma patients is independent of Breslow thickness, histologic type and tumor site. Ann Oncol 2008; 19: 583–589. https://doi.org/10.1093/annonc/mdm498
- Borkowska AM, Szumera-Ciećkiewicz A, Spałek MJ, Teterycz P, Czarnecka AM, Rutkowski P. Clinicopathological features and prognostic factors of primary acral melanomas in Caucasians. J Clin Med 2020; 9: 2996. https://doi.org/10.3390/jcm9092996
- Bian SX, Hwang L, Hwang J, Ragab O, In GK, Peng D, et al. Acral lentiginous melanoma-population, treatment, and survival using the NCDB from 2004 to 2015. Pigment Cell Melanoma Res 2021; 34: 1049–1061. https://doi.org/10.1111/pcmr.12999
- Joosse A, de Vries E, Eckel R, Nijsten T, Eggermont AM, Hölzel D, et al. Gender differences in melanoma survival: female patients have a decreased risk of metastasis. J Invest Dermatol 2011; 131: 719–726. https://doi.org/10.1038/jid.2010.354
- Bello DM, Chou JF, Panageas KS, Brady MS, Coit DG, Carvajal RD, et al. Prognosis of acral melanoma: a series of 281 patients. Ann Surg Oncol 2013; 20: 3618–3625. https://doi.org/10.1245/s10434-013-3089-0
- Teramoto Y, Keim U, Gesierich A, Schuler G, Fiedler E, Tüting T, et al. Acral lentiginous melanoma: a skin cancer with unfavourable prognostic features. A study of the German central malignant melanoma registry (CMMR) in 2050 patients. Br J Dermatol 2018; 178: 443–451. https://doi.org/10.1111/bjd.15803
- Susok L, Gambichler T. Caucasians with acral lentiginous melanoma have the same outcome as patients with stage- and limb-matched superficial spreading melanoma. J Cancer Res Clin Oncol 2022; 148: 497–502. https://doi.org/10.1007/s00432-021-03630-6
- Eriksson H, Frohm-Nilsson M, Järås J, Kanter-Lewensohn L, Kjellman P, Månsson-Brahme E, et al. Prognostic factors in localized invasive primary cutaneous malignant melanoma: results of a large population-based study. Br J Dermatol 2015; 172: 175–186. https://doi.org/10.1111/bjd.13171
- Statistical Database, Statistics Sweden. Available from: https://www.statistikdatabasen.scb.se/pxweb/sv/ssd/START__BE__BE0101__BE0101Q/UtlSvBakgGrov/table/tableViewLayout1/