SHORT COMMUNICATION
Pigmented Microcystic Adnexal Carcinoma with Melanocyte Colonization: A Case Report
Masakazu KAKURAI, Hanako MIYAHARA, Rie HONDA and Shusaku ITO
Division of Dermatology, Hitachi General Hospital, Hitachi General Hospital, 2-1-1 Jonan, Hitachi, Ibaraki 317-0077, Japan. E-mail: kakurai.masakazu.or@ms.hosp.tsukuba.ac.jp
Citation: Acta Derm Venereol 2024; 104: adv40329. DOI: https://doi.org/10.2340/actadv.v104.40329.
Copyright: © 2024 The Author(s). Published by MJS Publishing, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/).
Submitted: Mar 20, 2024; Accepted after revision: Jun 24, 2024; Published: Jul 15, 2024
INTRODUCTION
Microcystic adnexal carcinoma (MAC) is a rare malignant adnexal tumour with follicular and eccrine sweat gland differentiation (1–6). The most common clinical manifestation of MAC is a firm flesh-coloured nodule or plaque (2–6). Interestingly, pigmented MAC mimicking basal cell carcinoma (BCC), although rare, has been reported (2–6). However, the histological distribution of melanin deposits in pigmented MAC has not yet been described. We herein present a case of pigmented MAC with melanocyte colonization.
CASE REPORT
A 58-year-old Japanese man presented with a 3-year history of an asymptomatic nodule on his right upper lip that was slowly increasing in size. He was otherwise healthy and had no family history of skin tumours. A physical examination revealed a firm blue-grey nodule measuring 6 × 6 mm with a central depression and skin erosion on the right upper lip (Fig. 1a). Dermoscopy showed a centrally depressed nodule with blue-grey pigmentation, some yellowish-white clods and a linear vessel (Fig. 1b). Based on these features, a provisional diagnosis of BCC was considered and excisional biopsy was performed with a margin of 2 mm. Histologically, poorly circumscribed cords and nests of basaloid cells without atypia invaded the dermis, subcutaneous tissue and part of the muscle layer, consisting of keratin-filled cysts in the upper dermis as well as multiple cystic glands predominantly in the lower dermis (Fig. 1c, d). Tumour nests were surrounded by a fibrosclerotic stroma with the infiltration of some melanophages, and some melanin-containing cells were observed within tumour nests (Fig. 1d). Immunohistochemical staining with epithelial membrane antigen was positive for tumour cells, carcinoembryonic antigen was positive on the luminal surfaces of some eccrine ducts, and Ber-EP4 staining was negative for tumour cells (Fig. 1e–g). Melan-A and SRY-box transcription factor 10 (SOX10) were used to stain melanocytes. Melan-A was positive for dendritic and oval melanocytes within tumour nests (Fig. 1h), and the number of SOX10-positive melanocytes was increased among tumour cells (Fig. 1i, j); these cells were distributed evenly throughout the lesion as single cells (i.e. no melanocytic nests) and were devoid of atypia. Taken together, we corroborated the diagnosis of pigmented MAC. Although the tumour was clear of surgical margins in the initial excision, extended resection with an 8-mm margin was performed to prevent local recurrence. The patient is scheduled for long-term follow-up for possible recurrence of the lesion.
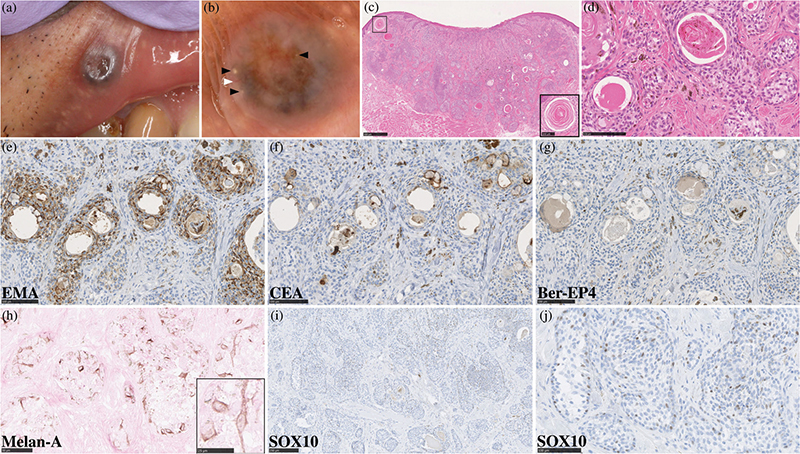
Fig. 1. Clinical and histological findings. (a) Clinical images of a firm blue-grey nodule, 6 × 6 mm in size, with a central depression and skin erosion on the right upper lip. (b) Dermoscopy showed a centrally depressed nodule with blue-grey pigmentation and some yellowish-white clods: black arrows, and a linear vessel: white arrow (×15). (c) A haematoxylin-eosin-stained slide of the blue-grey nodule revealed that in addition to dilated blood vessels in the upper dermis and skin erosion, poorly circumscribed cords and nests of basaloid cells invaded the dermis, subcutaneous tissue and part of the muscle layer, containing keratin-filled cysts in the upper dermis: black frame (bars in the large image = 500 μm; inset, bars = 100 μm). (d) Tumour nests comprised basaloid cells without atypia with multiple cystic glands, and tumour nests were surrounded by a fibrosclerotic stroma with the infiltration of some melanophages, and some melanin-containing cells were observed within tumour nests (bars = 100 μm). (e–g) Immunohistochemical staining with (e) epithelial membrane antigen and (f) carcinoembryonic antigen was positive for tumour cells and on some luminal surfaces of eccrine ducts, respectively, while (g) Ber-EP4 staining was negative for tumour cells (all bars = 100 μm). (h) Melan-A was positive for dendritic and oval melanocytes within tumour nests (bars = 50 μm; inset, bars = 25 μm). (i, j) SOX10-positive melanocytes without atypia were increased among tumour cells and were distributed evenly throughout the lesion (bars = 250 and 100 μm, respectively).
DISCUSSION
MAC is a rare malignant adnexal tumour with follicular and eccrine sweat gland differentiation that has a local aggressive growth pattern and rarely metastasizes (1–6). The incidence of MAC in the United States was 0.52 cases per 1,000,000 people per year, mostly affected Caucasians (83.3%), and was more prevalent in females (58%) (1). MAC generally presents as a firm flesh-coloured nodule or plaque in the head and neck region, particularly on the skin around the lip (2–6). However, in rare cases, pigmented MAC resembling BCC may occur (2–6). To the best of our knowledge, 5 cases of pigmented MAC have been reported to date (2–6), but the histological distribution of melanin deposits has not been investigated (2). We herein described a case of pigmented MAC with melanocyte colonization.
Melanocyte colonization within tumour nests has been reported in a number of benign and malignant skin tumours, such as seborrheic keratosis, BCC, pigmented eccrine porocarcinoma, pigmented breast carcinoma and rarely pigmented squamous cell carcinoma (7–13). In a previous case of pigmented MAC, melanocytes expressing S-100 were distributed among tumour cells (2), suggesting melanocyte colonization. Although the mechanisms underlying melanocyte colonization remain unknown, several hypotheses propose the involvement of tumour-released factors in melanocyte proliferation and melanin production. Lee et al. reported that melanocyte colonization, which was absent in areas of eccrine poroma, localized to eccrine porocarcinomatous areas within the same lesion, and this selective distribution suggested the promotion of melanocyte proliferation by malignancy-associated factors (7). Moreover, Saitoh et al. found that pigmented breast cancers produced basic fibroblast growth factor, which is a growth factor for melanocytes (8). Morisaka et al. subsequently suggested that endothelin-1 produced by breast cancer cells induces the overproduction of melanin in melanocytes (9). Furthermore, in benign skin tumours, including seborrheic keratosis, melanin may be transferred from melanocytes to tumour cells, resulting in the presence of melanin in tumour cells (10–12). In contrast, malignant skin tumours, such as BCC, pigmented eccrine porocarcinoma, pigmented breast carcinoma and pigmented squamous cell carcinoma, may have melanocytes with dysfunctional melanin transport from melanocytes to tumour cells, called melanin blockade melanocytes (11, 12). In the current case, staining with Melan-A and SOX10 revealed a similar number of positive cells for both stains, demonstrating melanocyte colonization. Therefore, blue-grey pigmentation in the current case corresponded to melanocyte colonization and increased melanophages. In addition, tumour cells did not uniformly contain melanin, as in seborrheic keratosis, suggesting that proliferating melanocytes in pigmented MAC have the characteristics of melanin blockade melanocytes.
To date, 5 out of 6 cases of pigmented MAC, including the current case, have been reported in Asian countries (2, 3, 5, 6). Age at onset ranged between 11 and 72 years (mean: 44 years), and predominantly affected males (83%) (2–6). Most cases occurred on the face and one on the scapular region (2–6). Dermoscopic findings of pigmented MAC reportedly include blue-grey to brown dots and globules as well as arborizing vessels, similar to the dermoscopic features of BCC (5, 6, 13). In the current case, the blue-grey nodule with a central depression and skin erosion resembled BCC, which is often pigmented in Asians (13). Therefore, clinicians need to be aware of a blue-grey to brown nodule or plaque resembling BCC as one of the clinical manifestations of MAC. While the number of pigmented MAC cases is too small to draw definitive conclusions, our literature review suggests that the development of pigmented MAC is more prevalent among Asians. The further accumulation of pigmented MAC cases is warranted to obtain a more detailed understanding of development of pigmented MAC in specific races and ethnicities.
REFERENCES
- Amon G, Liszewski W, Maher IA. Epidemiology and survival of microcystic adnexal carcinoma by sex in the United States. Dermatol Surg 2021; 47: 127-129. https://doi.org/10.1097/DSS.0000000000002167
- Lan XM, Zhong BY, Yan H, Feng L, Tan H, Yang XC. Diagnosis of pigmented microcystic adnexal carcinoma. G Ital Dermatol Venereol 2016; 151: 564-565.
- Yoshida Y, Shiomi T, Shindo M, Suyama Y, Nakayama B, Yamamoto O. A brownish-grey plaque with ulceration on the nasolabial area: a quiz. Microcystic adnexal carcinoma. Acta Derm Venereol 2012; 92: 109-110. https://doi.org/10.2340/00015555-1172
- Calderón-Castrat X, Román-Curto C, Santos-Briz A, Fernández-López E. Microcystic adnexal carcinoma mimicking basal cell carcinoma. JAAD Case Rep 2017; 3: 492-494. https://doi.org/10.1016/j.jdcr.2017.07.010
- Gupta V, Kakkar A, Agarwal S, Sulaiman M, Ramam M. Dermoscopic pitfall: microcystic adnexal carcinoma mimicking basal cell carcinoma. Indian J Dermatol Venereol Leprol 2020; 86: 202-205. https://doi.org/10.4103/ijdvl.IJDVL_209_19
- Pratiwi NI, Djawad K, Wijaya JK, Ghaznawie M, Wahab S, Nurdin A. A diagnostic challenge in an atypical variant of microcystic adnexal carcinoma mimicking ulcerative basal cell carcinoma: a case report and brief literature review. Acta Dermatovenerol Alp Pannonica Adriat 2022; 31: 151-155. https://doi.org/10.15570/actaapa.2022.26
- Lee HJ, Jeong SH, Seo EJ, Ha SJ, Kim JW. Melanocyte colonization associated with malignant transformation of eccrine poroma. Br J Dermatol 1999; 141: 582-583. https://doi.org/10.1046/j.1365-2133.1999.03070.x
- Saitoh K, Saga K, Okazaki M, Maeda K. Pigmented primary carcinoma of the breast: a clinical mimic of malignant melanoma. Br J Dermatol 1998; 139: 287-290. https://doi.org/10.1046/j.1365-2133.1998.02368.x
- Morisaka H, Nakajima R, Aoyama Y, Teraishi M, Nakajima H, Ogawa M, et al. Case of pigmented skin metastasis of breast carcinoma. J Dermatol 2021; 48: e476-e477. https://doi.org/10.1111/1346-8138.16026
- Morais PM, Schettini APM, Rocha JA, Silva Júnior RCD. Pigmented squamous cell carcinoma: case report and importance of differential diagnosis. An Bras Dermatol 2018; 93: 96-98. https://doi.org/10.1590/abd1806-4841.20186757
- Akasaka T. Clinicopathology of the pigmented epidermal tumors. Jpn J Dermatol 2002; 112: 1221-1228.
- Kilitci A, Elmas ÖF, Akdeniz N, Gamsızkan M. Melanocytes as the source of the increased melanisation in pigmented epithelial tumours: a holistic approach. Turk J Med Sci 2022; 52: 691-698. https://doi.org/10.55730/1300-0144.5362
- Behera B, Kumari R, Thappa DM, Gochhait D, Srinivas BH, Ayyanar P. Dermoscopic features of basal cell carcinoma in skin of color: a retrospective cross-sectional study from Puducherry, South India. Indian J Dermatol Venereol Leprol 2023; 89: 254-260. https://doi.org/10.25259/IJDVL_420_20