ORIGINAL REPORT
Mucocutaneous Manifestations in Patients with Dengue Fever: From the EPIDENGUE Cohort on Reunion Island
Olivier MAILLARD1,2#, Clotilde FERA3#, Elisa JOLY3, Kevin DIALLO3, Patrick MAVINGUI4, Yves-Marie DIARRA1,4, Yatrika KOUMAR3, André CABIÉ5,6 and Antoine BERTOLOTTI1,3
1Clinical Investigation Center, INSERM CIC1410, University Hospital of Reunion, Saint Pierre, Reunion, 2Department of Public Health and Research, University Hospital of Reunion, Saint-Pierre, Reunion, 3Department of Infectious Diseases and Dermatology, University Hospital of Reunion, Saint-Pierre, Reunion, 4UMR PIMIT, CNRS 9192, INSERM 1187, IRD 249, University of Réunion, Sainte-Clotilde, Reunion, 5Department of Infectious Diseases, University Hospital of Martinique, Fort-de-France, Martinique, 6Clinical Investigation Center, Inserm CIC1424, University Hospital of Martinique, Fort-de-France, Martinique, France
#These authors contributed equally to this work
Nearly 4 billion people live in a dengue risk area worldwide. The prevalence of dengue-related mucocutaneous manifestations and their association with severe dengue differ across studies. The aim of the study was to describe the characteristics of patients with dengue-related mucocutaneous manifestations and to investigate those were associated with severe dengue. A retrospective study was conducted in 2019 among patients with a positive RT-PCR for dengue at the University Hospital of Reunion, which has been experiencing a re-emergence of dengue since 2018. Of 847 patients with confirmed dengue, 283 (33.4%) developed mucocutaneous manifestations. Only manifestations of dehydration such as glossitis, dysgeusia, or conjunctivitis were associated with severe dengue, unlike pruritus and rash, in bivariate analysis but not in multivariate analysis. The rash and pruritus of dengue appear to be accompanied by a pronounced flu-like syndrome in younger people without comorbidity or severity, although careful examination of mucous membranes would better identify signs of dehydration and thus cases likely to worsen.
Key words: dengue fever; skin manifestations; reunion.
SIGNIFICANCE
Nearly 4 billion people live in a dengue risk area worldwide. The prevalence of dengue-related mucocutaneous manifestations and their association with severity of dengue differ across studies. In our setting, a third of dengue patients developed mucocutaneous manifestations with no difference regarding severity of dengue. Mucocutaneous manifestations were associated with dizziness and dehydration. The rash and pruritus of dengue appear to be accompanied by a pronounced flu-like syndrome in younger people without the medical background or severity of dengue, although careful examination of mucous membranes would better identify signs of dehydration and thus cases likely to worsen.
Citation: Acta Derm Venereol 2024; 104: adv40334. DOI https://doi.org/10.2340/actadv.v104.40334.
Copyright: © 2024 The Author(s). Published by MJS Publishing, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/).
Submitted: Mar 13, 2024; Accepted after revision: Jun 24, 2024; Published: Jul 18, 2024
Corr: Olivier Maillard, INSERM CIC 1410, Centre Hospitalier Universitaire de La Réunion, BP 350 – 97448 Saint Pierre Cedex, Reunion Island, France. E-mail: olmaillard@yahoo.fr
Competing interests and funding: The authors have no conflict of interest to declare.
This work was supported by the European Regional Development Fund through the RUNDENG project No.20202640-0022937. The fund had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
INTRODUCTION
Dengue fever, caused by the dengue virus transmitted through Aedes mosquitoes, remains a global health threat, particularly in tropical and subtropical regions. With approximately 390 million infections reported annually, it poses significant socio-economic burdens and challenges to healthcare systems worldwide (1). This arboviral disease manifests as a spectrum of clinical presentations, from mild flu-like symptoms to severe haemorrhagic fever and organ failure, often leading to fatalities (2, 3).
Apart from its classic symptoms such as high fever, headache, retro-orbital pain, joint and muscle pain, and back pain, dengue often presents with distinctive mucocutaneous manifestations. The scientific literature provides valuable insights into the various mucocutaneous manifestations associated with dengue fever, emphasizing the importance of considering these symptoms in clinical diagnosis (4–6). Additionally, the correlation between specific skin findings and the progression of the disease sheds light on potential indicators of severe dengue (6).
Reunion Island, a French overseas territory in the South-West Indian Ocean, has been experiencing a re-emergence of dengue since 2018 (7). In 2019, 18,217 confirmed cases were notified to the island’s surveillance system (75% in the south) for an estimated 42,420 (5% of the population), of which 1,944 were admitted to emergency units and 620 were hospitalized. Only 22 were secondary dengue cases, and 14 deaths were linked to dengue (8).
The aim of this study was to describe the characteristics of dengue patients who developed mucocutaneous manifestations in 2019 on Reunion Island and to investigate those associated with severe dengue.
MATERIALS AND METHODS
The study was based on a retrospective cohort nested in the EPIDENGUE database, which involved patients who were suspected of having dengue fever between January and June 2019 in emergency units of the University Hospital of Reunion. The EPIDENGUE database was set up to study dengue fever in Reunionese patients first during the 2019 epidemic, while a prospective study was launched at the same time but with limited recruitment because emergency services were overwhelmed during the epidemic (9). Confirmation of the diagnosis of dengue fever was limited to RT-PCR-positive patients, as IgM serology was not systematically double checked, and NS1 ELISA was not undertaken routinely. Moreover, NS1 antigen rapid diagnostic tests have been shown to perform poorly in a previous study (10). Probable and severe dengue were defined according the 2009 WHO definition (2). A probable dengue case is a patient with fever and at least 2 clinical signs of dengue. Severe dengue (SD) was defined by the occurrence of plasma leakage that may lead to shock and/or fluid accumulation, with or without respiratory distress, and/or severe bleeding, and/or severe organ impairment. The severe dengue variable was coded in the analysis phase using the 2009 WHO definition. The coding was validated by 3 physicians and a pharmacist trained on data from the prospective database (11), where the diagnosis of severe dengue was made for each patient. Clinical and biological data were collected from patients’ medical files from admission until 10 days of management for hospitalized patients (except death status, which was reported at the time of the collection of data in 2020).
The pruritus variable included the occurrence of pruritus in any body region. The erythematous rash variable included diffuse erythema that covered more than 50% of the body surface, maculo-papular erythema that covered less than 50% of the body, and palmoplantar erythema. Mouth involvement included lip, tongue, cheek, angular cheilitis, pharyngitis, mouth ulcer, and gingivitis. The mucocutaneous manifestations variable included all the variables collected. Mucosal bleeding included mucocutaneous bleeding: skin, conjunctivitis, epistaxis, bleeding gums, and other haemorrhagic signs: metrorrhagia, menorrhagia, gastro-intestinal bleeding (rectal bleeding, melaena), and haematuria.
In descriptive analyses, means and standard deviations were used for categorical variables; medians, interquartile ranges, and ranges were used for quantitative variables. In bivariate analyses, a χ2 test or Fisher’s exact test was used for categorical variables; Student’s t-test or a non-parametric Mann–Whitney test was used for quantitative variables. In multivariate analyses, a backward stepwise procedure was used to perform a binary binomial logistic regression, including variables with p < 0.20 in bivariate analyses. Adjusted odds ratios (OR) and their 95% confidence intervals (95%CI) were derived from the regression coefficients. All tests were two-tailed and the significance level was set at 0.05. SPSS software (IBM SPSS 23.0; IBM Corp, Armonk, NY, USA) was used for statistical analysis.
In accordance with French regulations, this monocentric observational retrospective study of data re-use did not require an Ethics Committee (article R1121-1, decree no. 2017-884 of 9 May 2018 - art.2), but the ethical character of the study was approved by the Institutional Review Board of the University Hospital of Reunion and the EPIDENGUE database was registered in the National Health Data Hub (no. F20201021104344). This study was conducted according to the Declaration of Helsinki and the reference methodology MR-004 for research not involving the human person (registration number 2206739) of the National Commission for Information Technology and Liberties (CNIL), which is in line with the General Data Protection Regulation (GDPR) of the European Union. Agreement to non-refusal participation was obtained from participants and data were treated anonymously.
RESULTS
A positive RT-PCR was retrieved in 847 of the 2,365 patients included in the EPIDENGUE cohort. Mucocutaneous manifestations were varied and were reported in 283 (33.40%) of them (Figs 1 and 2).
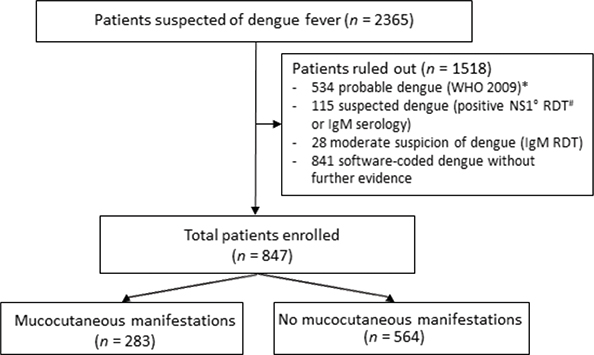
Fig. 1. Flowchart, Reunion 2019. *World Health Organization. Dengue guidelines for diagnosis, treatment, prevention and control: new edition. World Health Organization, 2009. Report No.: WHO/HTM/NTD/DEN/2009.1. Available from: https://apps.who.int/iris/handle/10665/44188. #RDT: rapid diagnostic test. °NS1: dengue NS1 antigen.
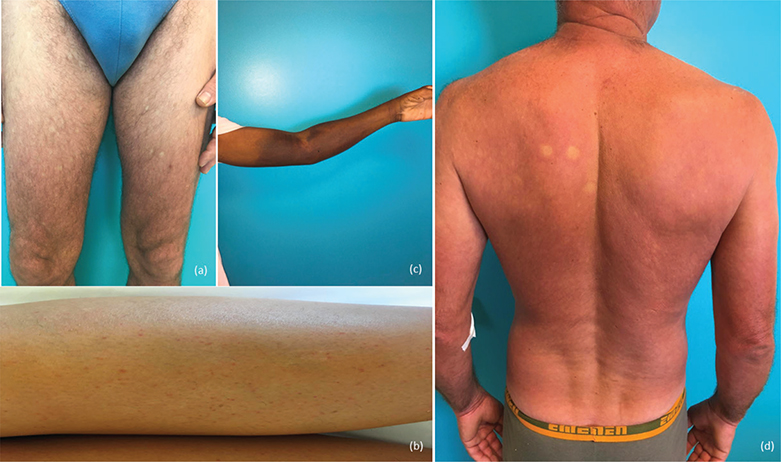
Fig. 2. (A) Confluent diffuse macular eruption with “white islands in a red sea”; (B) purpura of the leg; (C) hematoma after blood test in a patient with a thrombocytopenia; (D) diffuse erythematous rash with finger marks.
There was no significant difference in the occurrence of mucocutaneous manifestations when comparing severe vs non severe dengue, (30.9% vs 34.0%, p = 0.447). However, a significant association in favour of severe dengue was identified for tongue manifestations (3.6% vs 0.9%, p = 0.007) and dysgeusia (11.5% vs 6.6%, p = 0.032), as well as conjunctivitis (3.0% vs 0.9%, p = 0.044) compared with non-severe dengue (Table I).
| Factor | Non severe dengue (n = 682) n (%) | Severe dengue (n = 165) n (%) | p-value# |
| Mucocutaneous manifestations*, n = 283 | 232 (34.0) | 51 (30.9) | 0.447 |
| Pruritus: | 47 (6.9) | 13 (7.9) | 0.657 |
| Hand pruritus | 22 (3.2) | 3 (1.8) | 0.447 |
| Feet pruritus | 20 (2.9) | 3 (1.8) | 0.596 |
| Trunk pruritus | 20 (2.9) | 6 (3.6) | 0.638 |
| Face pruritus | 16 (2.3) | 4 (2.4) | 1.000 |
| Lower limbs pruritus | 22 (3.2) | 8 (4.8) | 0.312 |
| Upper limbs pruritus | 23 (3.4) | 6 (3.6) | 0.867 |
| Rash: | 102 (15.0) | 21 (12.7) | 0.466 |
| White islands | 6 (0.9) | 1 (0.6) | 1.000 |
| Morbilliform | 26 (3.8) | 9 (5.5) | 0.342 |
| Scarlatiniform | 4 (0.6) | 0 (0.0) | 1.000 |
| Papular | 15 (2.2) | 4 (2.4) | 0.775 |
| Face | 23 (3.4) | 5 (3.0) | 0.825 |
| Back | 44 (6.5) | 6 (3.6) | 0.169 |
| Abdomen | 40 (5.9) | 11 (6.7) | 0.698 |
| Upper limbs | 32 (4.7) | 8 (4.8) | 0.932 |
| Lower limbs | 35 (5.1) | 8 (4.8) | 0.882 |
| Palmo-plantar erythema: | 4 (0.6) | 1 (0.6) | 1.000 |
| Diffuse rash | 48 (7.0) | 7 (4.2) | 0.191 |
| Vesicular rash | 7 (1.0) | 0 (0.0) | 0.357 |
| Bullous rash | 1 (0.1) | 0 (0.0) | 1.000 |
| Hand oedema | 0 (0.0) | 1 (0.6) | 0.195 |
| Feet oedema | 3 (0.4) | 2 (1.2) | 0.252 |
| Other oedema | 6 (0.9) | 0 (0.0) | 0.603 |
| Mouth involvement: | 74 (10.9) | 24 (14.5) | 0.183 |
| Cheilitis | 20 (2.9) | 8 (4.8) | 0.217 |
| Glossitis | 6 (0.9) | 6 (3.6) | 0.007 |
| Stomatitis | 1 (0.1) | 1 (0.6) | 0.352 |
| Angular cheilitis | 1 (0.1) | 0 (0.0) | 1.000 |
| Mucositis | 21 (3.1) | 5 (3.0) | 0.974 |
| Mouth ulcer | – | – | – |
| Gingivitis | 4 (0.6) | 1 (0.6) | 1.000 |
| Conjunctivitis | 6 (0.9) | 5 (3.0) | 0.044 |
| Haemorrhagic signs: | 67 (9.7) | 14 (9.1) | |
| Gingivorrhagia | 25 (3.7) | 5 (3.0) | 0.692 |
| Petechial purpura | 29 (4.3) | 8 (4.8) | 0.737 |
| Ecchymotic purpura | 1 (0.1) | 2 (1.2) | 0.099 |
| Conjunctival hyperaemia | 2 (0.3) | 0 (0.0) | 1.000 |
| Epistaxis | 17 (2.5) | 2 (1.2) | 0.556 |
| Dyschromia | 1 (0.1) | 0 (0.0) | 1.000 |
| Auricular pseudochondritis | 0 (0.0) | 1 (0.6) | 0.195 |
| Acrosyndrome | 0 (0.0) | 1 (0.6) | 0.195 |
| Jaundice | 4 (0.6) | 2 (1.2) | 0.332 |
| Other mucocutaneous signs* | 7 (1.0) | 1 (0.6) | 1.000 |
| Hyperesthesia | 8 (1.2) | 4 (2.4) | 0.263 |
| Dysgeusia | 45 (6.6) | 19 (11.5) | 0.032 |
| #Pearson’s χ2 test and Fisher’s exact test; p-values in bold are statistically significant. | |||
| *Other mucocutaneous signs: cyanosis, vulvar vesicular eruption, erysipelas, eyelid oedema, groin intertrigo, soft palate purpura. | |||
In multivariate analysis (Table II), patients presenting with mucocutaneous manifestations during dengue fever were significantly younger (p < 0.001) and had more pain (p = 0.001), required rehydration (p < 0.001), and had dizziness (p < 0.001), but less frequently had diabetes (p = 0.045) or profound thrombocytopenia (p < 0.001). When focusing on patients with erythematous rash in a multivariate model (Table III), they were also younger (p < 0.001), experienced more pruritus (p < 0.001), and had more pain (p = 0.045), but less frequently presented with diabetes (p = 0.039) or profound thrombocytopenia (p < 0.001). The presence of pruritus was more closely correlated with female gender (p = 0.030), dizziness (p < 0.001), maximum sodium level (p = 0.021), and erythematous rash (p < 0.001). The other characteristics of the study population are provided in Table SI with the bivariate analysis according to the occurrence of mucocutaneous events during dengue fever.
| Erythematous rash* n = 132 | Pruritus n = 56 | |||||
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Age | 0.973 | 0.962–0.984 | < 0.001 | – | – | – |
| Diabetes | 0.446 | 0.208–0.958 | 0.039 | – | – | – |
| Platelets (G/L), min | 0.993 | 0.990–0.997 | < 0.001 | – | – | – |
| Pain | 1.728 | 1.012–2.952 | 0.045 | – | – | – |
| Pruritus | 6.993 | 3.841–12.732 | < 0.001 | – | – | – |
| Dizziness | – | – | – | 4.657 | 2.231–9.718 | < 0.001 |
| Sodium (mmol/L), max | – | – | – | 1.120 | 1.017–1.233 | 0.021 |
| Erythematous rash | – | – | – | 9.518 | 5.132–17.654 | < 0.001 |
| Female sex | – | – | – | 2.222 | 1.080–4.572 | 0.030 |
| Pregnancy | – | – | – | 3.099 | 1.402–6.849 | 0.005 |
| *Erythematous rash: include diffuse rash, maculo-papular rash, palmo-plantar erythema. | ||||||
| OR: odds ratio; 95% CI: 95% confidence interval. | ||||||
Considering the mucosal manifestations in particular, patients with mouth involvement were younger but affected by a severe flu-like syndrome, diarrhoea, cough, dizziness, hypernatremia, or the need for rehydration (Table SII). Mucosal bleeding was associated with younger age, female gender, dizziness, diarrhoea, lower minimum platelet counts, and increased haematocrit (Table SIII).
Finally, multivariate analysis in patients with mucocutaneous manifestations showed an increased risk of severe dengue in those with palpitations, cancer, anti-platelet agents, anticoagulants, and requiring rehydration (Table SIV).
DISCUSSION
This retrospective study retrieved 33.4% with mucocutaneous manifestations and did not reveal any significant association between those events and the development of severe dengue. However, mucosal damage involving the tongue or conjunctiva, and dysgeusia could be indicative of severe dengue. Studies on this subject are contradictory in the literature. Even if bleeding and obviously severe bleeding as defined by the WHO (2) are reported to be associated with SD in 2 recent meta-analyses, which have included 87 and 122 studies (11, 12), only melaena (OR = 4.05; 95% CI 1.64–10.00, p < 0.001) is reported to be independently associated in a meta-analysis conducted in 143 studies (14). In addition, epistaxis (OR 2.23, 95% CI 1.04–4.77, p = 0.04), gum bleeding (OR 3.34, 95% CI 1.60–6.98, p < 0.01), and skin bleeding (OR 2.12, 95% CI 1.53–3.19, p < 0.001) were found to be associated with SD in another meta-analysis of 39 studies (15). In India in 2018, 387 patients were studied prospectively and no significant difference was found in the development of severe dengue within the groups classified by rash (erythema or petechiae) or no rash. However, patients with rash were more likely to have low platelets and non-severe haemorrhagic manifestations (mucocutaneous bleeding, epistaxis, haematuria) (6).
In multivariate analysis, patients affected by dengue with mucocutaneous manifestations or only erythematous rash appeared to be younger, without comorbidities, but with a much more pronounced and painful flu-like syndrome and the need for more rehydration. The skin lesions would probably be related to a host immunopathological process releasing cutaneous chemical mediators during mucocutaneous manifestations (15, 16). Younger, healthy patients would therefore react more to the virus than older subjects. Furthermore, dehydration during the acute phase of arboviruses seems to lead patients towards a severe form of the disease (17–19).
Rash could also be a direct clinical sign of DENV virus replication, as has been observed in other severe viral infections, including measles and pox virus skin lesions (17). Morsy et al. (21) reported that viremia is strongly associated with disease severity and type of infection (primary or secondary) as well as with time from first symptom to consultation and dengue serotype with higher viremia in DENV-2/1, which co-circulated in Reunion in 2019 (11). Skin rash could then be an indicator of SD, which needs to be studied in greater depth and prospectively on a sufficient number of subjects.
The main limitations of this work are those related to the retrospective design. Notably, there is a lack of information on the chronology of mucocutaneous manifestations and SD to assess whether some of them might be predictive of SD. Furthermore, due to the absence of a second serology test at 3 weeks and a lack of diagnostic accuracy of the NS1 Ag rapid diagnostic test used in 2019 at the University Hospital of Reunion (10), only RT-PCR was used to confirm a dengue diagnosis (2), and viremia was not routinely tested for.
In a prospective study undertaken at the same time and in the same setting but on fewer patients (n = 163) (9), mucocutaneous manifestations were reported in higher proportion (80%), but they were not associated with severe dengue, except for ecchymotic purpura in bivariate analysis (p = 0.037). This difference may be explained by the retrospective recruitment without systematic follow-up, but also highlights that skin signs are not sufficiently considered during clinical examination by non-dermatologists, with a systematic skin examination reported by less than 30% of primary care physicians (22). In the literature, dengue-related skin and mucous membrane disorders are common, affecting 50–82% of patients (23).
An initial fleeting rash may occur within the first few hours of symptoms (4), but patients who consulted after 48 h and phototypes IV and V are predominant in Reunion Island, thus limiting its observation by a doctor. Furthermore, 46% of the cohort were hospitalized so outpatients may have presented with mucocutaneous manifestations after their consultation that were not reported.
The rash and pruritus in dengue fever appear to be accompanied by a pronounced flu-like syndrome in younger people without comorbidity or severity, but careful examination of the mucous membranes would help to identify signs of dehydration and therefore cases that could worsen. Further prospective studies with a consistent sample size are needed to fully assess the incidence, timing, and duration of mucocutaneous manifestations in dengue patients and to identify those that may be associated with severe dengue.
ACKNOWLEDGEMENTS
Tahe authors would like thank Drs E. Antok, Q. Balacheff, E. Barange, J.P. Becquart, L. Brochet, R. Chane Teng, M. Crouzet, A.B. Da Silva Gomez, C. Daubard, A. Devergez, D. Khemiri, K. Larsen, M. Lemeur, L. Raffray, M. Ruin, F. Tixier, N. Ebran, B. Fontaine, C. Hebert, D. Hirschinger, E. Huchot, E, Jarlet, H. Flodrops, M. Lafon, A. Laval, O. Lamouret, J. Lemant, J.C. Maiza, R. Manaquin, P. Poubeau, R. Perrin, A. Plantier, C. Schweizer, L. Thibault, O. Meilhac, A. Issop, J. Belot, M. Legros, M. Carras, A.C. Etoa, M. Cadic, M. Randriamanana, J Jaubert, C Rosolen, E Magny, Y.M. Diarra, P. Gerardin, and C. Marimoutou. They would also like to thank V. Grondin, J. Jean-Marie, I. Calmont, and J. Ruiz.
REFERENCES
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature 2013; 496: 504-507. https://doi.org/10.1038/nature12060
- World Health Organization. Dengue: Guidelines for diagnosis, treatment, prevention and control: New Edition. Geneva: WHO, 2009 (cited 2024 Jun 17). Available from: http://www.ncbi.nlm.nih.gov/books/NBK143157/
- Guzman MG, Gubler DJ, Izquierdo A, Martinez E, Halstead SB. Dengue infection. Nat Rev Dis Primers 2016; 2: 16055. https://doi.org/10.1038/nrdp.2016.55
- Waterman SH, Gubler DJ. Dengue fever. Clin Dermatol 1989; 7: 117-122. https://doi.org/10.1016/0738-081X(89)90034-5
- Braun M, Andersen LK, Norton SA, Coates SJ. Dengue: updates for dermatologists on the world's fastest-growing vector-borne disease. Int J Dermatol 2023; 62: 1110-1120. https://doi.org/10.1111/ijd.16739
- Mishra AK, George AA, Abhilash KPP. The relationship between skin rash and outcome in dengue. J Vector Borne Dis 2018; 55: 310-314. https://doi.org/10.4103/0972-9062.256567
- Hafsia S, Haramboure M, Wilkinson DA, Baldet T, Yemadje-Menudier L, Vincent M, et al. Overview of dengue outbreaks in the southwestern Indian Ocean and analysis of factors involved in the shift toward endemicity in Reunion Island: a systematic review. PLoS Negl Trop Dis 2022; 16: e0010547. https://doi.org/10.1371/journal.pntd.0010547
- Santé Publique France. Surveillance de la dengue à La Réunion. Point au 7 décembre 2021 (Internet) [cited 2024 Jun 17]. Available from: https://www.santepubliquefrance.fr/regions/ocean-indien/documents/bulletin-regional/2021/surveillance-de-la-dengue-a-la-reunion.-point-au-7-decembre-2021
- Fera C, Maillard O, Joly E, Diallo K, Mavingui P, Koumar Y, et al. Descriptive and comparative analysis of mucocutaneous manifestations in patients with dengue fever: a prospective study. J Eur Acad Dermatol Venereol 2024 38: 191-196. https://doi.org/10.1111/jdv.19453
- Maillard O, Belot J, Adenis T, Rollot O, Adenis A, Guihard B, et al. Early diagnosis of dengue: diagnostic utility of the SD BIOLINE Dengue Duo rapid test in Reunion Island. PLoS Negl Trop Dis 2023; 17: e0011253. https://doi.org/10.1371/journal.pntd.0011253
- Carras M, Maillard O, Cousty J, Gérardin P, Boukerrou M, Raffray L, et al. Associated risk factors of severe dengue in Reunion Island: a prospective cohort study. PLoS Negl Trop Dis 2023; 17: e0011260. https://doi.org/10.1371/journal.pntd.0011260
- Yuan K, Chen Y, Zhong M, Lin Y, Liu L. Risk and predictive factors for severe dengue infection: a systematic review and meta-analysis. PLoS One 2022; 17: e0267186. https://doi.org/10.1371/journal.pone.0267186
- Sangkaew S, Ming D, Boonyasiri A, Honeyford K, Kalayanarooj S, Yacoub S, et al. Risk predictors of progression to severe disease during the febrile phase of dengue: a systematic review and meta-analysis. Lancet Infect Dis 2021; 21: 1014-1026. https://doi.org/10.1016/S1473-3099(20)30601-0
- Tsheten T, Clements ACA, Gray DJ, Adhikary RK, Furuya-Kanamori L, Wangdi K. Clinical predictors of severe dengue: a systematic review and meta-analysis. Infect Dis Poverty 2021; 10: 123. https://doi.org/10.1186/s40249-021-00908-2
- Htun TP, Xiong Z, Pang J. Clinical signs and symptoms associated with WHO severe dengue classification: a systematic review and meta-analysis. Emerg Microbes Infect 2021; 10: 1116-1128. https://doi.org/10.1080/22221751.2021.1935327
- de Andino RM, Botet MV, Gubler DJ, García C, Laboy E, Espada F, et al. The absence of dengue virus in the skin lesions of dengue fever. Int J Dermatol 1985; 24: 48-51. https://doi.org/10.1111/j.1365-4362.1985.tb05360.x
- King CA, Wegman AD, Endy TP. Mobilization and activation of the innate immune response to dengue virus. Front Cell Infect Microbiol 2020; 10: 574417. https://doi.org/10.3389/fcimb.2020.574417
- Harris E, Pérez L, Phares CR, Pérez M de los A, Idiaquez W, Rocha J, et al. Fluid intake and decreased risk for hospitalization for dengue fever, Nicaragua. Emerg Infect Dis 2003; 9: 1003-1006. https://doi.org/10.3201/eid0908.020456
- Lee I-K, Lee W-H, Yang KD, Liu J-W. Comparison of the effects of oral hydration and intravenous fluid replacement in adult patients with non-shock dengue hemorrhagic fever in Taiwan. Trans R Soc Trop Med Hyg 2010; 104: 541-545. https://doi.org/10.1016/j.trstmh.2010.05.003
- Besnard O, Maillard O, Franco J-M, Lebreton N, Reix G, Legrand F, et al. Hydration and clinical warning signs of dengue fever in primary care: an observational prospective study. Infect Dis Now 2023; 53: 104708. https://doi.org/10.1016/j.idnow.2023.104708
- Morsy S, Hashan MR, Hieu TH, Mohammed AT, Elawady SS, Ghosh P, et al. The association between dengue viremia kinetics and dengue severity: a systemic review and meta-analysis. Rev Med Virol 2020; 30: 1-10. https://doi.org/10.1002/rmv.2121
- Lowell BA, Froelich CW, Federman DG, Kirsner RS. Dermatology in primary care: pPrevalence and patient disposition. J Am Acad Dermatol 2001; 45: 250-255. https://doi.org/10.1067/mjd.2001.114598
- Thomas EA, John M, Kanish B. Mucocutaneous manifestations of dengue fever. Indian J Dermatol 2010; 55: 79-85. https://doi.org/10.4103/0019-5154.60359