SHORT COMMUNICATION
Long-term Remission of Severe and Refractory Chronic Actinic Dermatosis with Dupilumab: A Case Report with Review of the Literature
Clélia VANHAECKE1 and Manuelle VIGUIER2*, for the Société Française de Photodermatologie
1Dermatology department, Robert Debré Hospital, Reims University Hospital, Reims, France, and 2Reims Champagne-Ardenne University, Dermatology department, Reims University Hospital, IRMAIC EA7509, Reims, France. *E-mail: mviguier@chu-reims.fr
Citation: Acta Derm Venereol 2024; 104: adv40453. DOI: https://doi.org/10.2340/actadv.v104.40453.
Copyright: © 2024 The Author(s). Published by MJS Publishing, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/).
Submitted: Mar 29, 2024; Accepted after revision: Jul 29, 2024. Published: Aug 23, 2024
INTRODUCTION
Chronic actinic dermatitis (CAD) is a rare photodermatosis characterized by a chronic eczematous eruption. It includes several photodermatoses reported with different denominations in the international literature, such as persistent light reaction, photosensitive eczema and actinic reticuloid (1). Chronis actinic dermatitis initially involves sun-exposed areas of the skin and secondarily is diffuse, with predominance on sun-exposed areas (2). It classically occurs in older men and has a significant negative impact on quality of life (3). The aetiology is still unknown; the predominant hypothesis is a delayed-type hypersensitivity response (type IV) induced by skin antigens. The role of T helper 2 cell (Th2) immunity has been reported (4). A correlation between CAD and increased sun exposure has been suggested in Korean patients. Photoallergy and contact allergy are frequently found, with CAD clinical severity correlating with the total IgE level and CCR4 expression (5). Common treatments include strict photoprotection, topical corticosteroids or topical calcineurin inhibitors, hydroxychloroquine, and systemic immunosuppressants such as systemic corticosteroids, azathioprine, ciclosporin, and thalidomide (1). However, significant skin improvement is rare and immunosuppressive treatments can induce severe adverse effects, particularly in older patients.
Dupilumab is an interleukin 4 receptor α antagonist approved for treating several diseases characterized by predominant Th2-type immune reaction, including atopic dermatitis, with favourable tolerance particularly in older atopic patients (6). Several case reports have suggested the efficacy of dupilumab in CAD (7–14).
Here we report the rapid and durable efficacy of dupilumab in an older man with CAD resistant to several immunosuppressant therapies and review the literature for other cases of CAD treated with dupilumab.
CASE REPORT
An 80-year-old man presented to our dermatology unit with an itchy, erosive, and scaly erythema on the face, neck, chest, arms, hands, and legs (Fig. 1). The eruption had started 1 year earlier. He had no personal or family history of atopic disease. Because of cardiovascular events, he was taking simvastatin, candesartan, and aspirin. Blood tests revealed major eosinophilia (9 x 109/L; normal < 0.5 x 109/L) and elevated total serum immunoglobulin E level (348 UI/mL; normal < 114 UI/mL). A skin biopsy showed epidermal parakeratosis and spongiosis. A perivascular lympho-histiocytic infiltrate was reported with associated scattered eosinophils. Immunohistochemistry revealed predominant CD4+CD7+ lymphocytes without clonal T lymphocytes in the skin or blood. Malignancy investigations, including CT scan, were negative. Contact eczema was initially suspected because of diclofenac gel application on the hands. Despite diclofenac gel and topical corticosteroid treatment, the eruption relapsed, with clear predominance on sun-exposed sites and pruritus associated with insomnia.
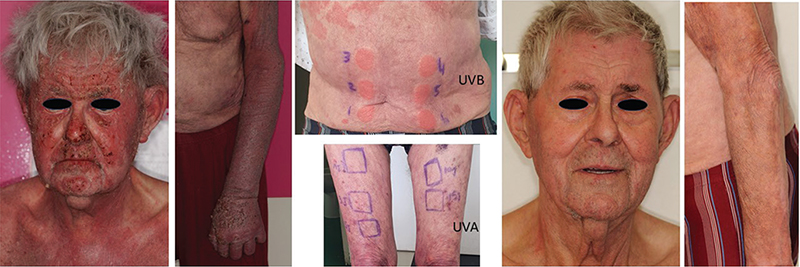
Fig. 1. Itchy, erosive and scaly erythema and complete remission 3 months after dupilumab initiation. Phototest results (for UVB light: 1=0.1 J/cm2; 2=0.12 J/cm2; 3=0.14 J/cm2; 4=0.16 J/cm2; 5=0.18 J/cm2; 6=0.20 J/cm2) revealed reduced minimal erythema dose to UVB light and normal minimal erythema dose to UVA light.
CAD was suspected, and phototests revealed substantially reduced minimal erythema dose (MED) to UVB light (< 0.1 J/cm2; normal >0.2) and normal MED to UVA light (Fig. 1). Patch tests gave positive results for cobalt chloride, nickel sulphate, formaldehyde, and Fragrance Mix II. The diagnosis of CAD was established, limited sun exposure was recommended, and successive treatments with azathioprine (2 months), hydroxychloroquine (3 months), then ciclosporin for 4 months conferred limited skin and general improvement. Additionally, azathioprine was associated with asthenia and anorexia. Dupilumab was initiated with an initial dose of 600 mg followed by 300 mg every other week. Three months later, a significant remission of the eruption (Fig. 1) and pruritus was noted. Blood level eosinophil count returned to the normal range (0.42 x 109/L; normal < 0.5 x 109/L). After 12 months, dupilumab was still being maintained at 300 mg every other week, with no adverse effects and with prolonged efficacy.
DISCUSSION
We confirmed the efficacy and safety of dupilumab for treating CAD in our case, with rapid and prolonged improvement. The rationale for using dupilumab in CAD is based on a clinical and pathological presentation suggesting eczema and a proven shift toward Th2 immunity from the Th2/Th1 balanced status in severe CAD (4).
Including our patient, 30 cases of CAD treated with dupilumab have been reported (Table SI), with a predominance of males (80%) and mean age 58.2 years, as expected in CAD (1). Of note, 21 (70%) of the reported patients were of Chinese origin (9, 12–14). Although CAD is classically described in older white men, as in our case, increasing cases have been reported in younger patients with darker skin types, particularly South Asians (14).
Arguments for associated atopic features in these patients are limited. Six (24%) of the 25 patients in the literature with available information had a history of atopic disease, mostly atopic dermatitis. The presence of eosinophilia was mentioned in 2 of 5 cases with documented hemograms, and 4 patients exhibited high or limited increased amounts of immunoglobulin E in serum, although the level was normal in 2. Nevertheless, all 4 patients with documented skin biopsies had spongiotic dermatitis.
Almost all patients showed a great decrease in MED in the UVB wavelength, as is frequently observed in CAD (1), sometimes associated with a decrease in MED in the UVA wavelength; only 1 patient also had decreased MED in visible light.
In all cases but one, dupilumab was administrated at least as second-line systemic treatment. A few patients (n = 7, 23%) had only received hydroxychloroquine before dupilumab; all others had received and showed failure to one or to several immunomodulatory or immunosuppressive drugs, including systemic corticosteroids (n = 12, 40%), methotrexate (n = 9; 30%), ciclosporin (n = 8; 27%), mycophenolate mofetil (n = 7; 23%), azathioprine (n = 5), thalidomide (n = 2), or apremilast (n = 1).
Dupilumab was always started at the usual dosage used in atopic dermatitis (loading dose of 600 mg, then 300 mg every other week), frequently associated with topical corticosteroids or topical tacrolimus. Dupilumab was started as a single systemic treatment in 14 patients (47%), together with hydroxychloroquine in 11 (37%), ciclosporin in 2 patients, or corticosteroids or thalidomide in 1 patient each. In 1 patient, methotrexate 15 mg/week was added to dupilumab after 8 months of monotherapy.
Altogether, with high publication bias, only 3 patients (10%) did not show CAD remission with dupilumab; 13 patients (43%) showed complete or almost complete remission of the photodermatosis and 14 patients (47%) significant alleviation of the disease. Clinical response was quickly obtained, with a mean delay of 1.8 months (range 1–4). Two of 3 patients who had phototests during dupilumab treatment showed limited improvement in UV tolerance, but none achieved complete clinical control (5, 8).
Of note, tolerance was similar to that observed with dupilumab in atopic dermatitis with several cases of blepharoconjunctivitis (n = 3) or facial redness (n = 1) (7, 8). One of the patients with blepharoconjunctivitis had a history of atopic dermatitis (8), but atopy history was unknown in the others.
Follow-up for more than 12 months was reported in 7 cases, including ours (7, 10, 11), with persistent response leading to spacing dupilumab injections every 4 weeks for 1 patient.
Dupilumab efficacy has been reported in photodermatoses other than CAD, such as solar urticaria (15) and actinic prurigo (16).
Altogether, administration of dupilumab in patients with CAD, with or without atopic history, seems rapidly effective and well tolerated in the long term, which allows for the withdrawal of immunosuppressants. Because of high publication bias, an open-labelled, prospective, proof-of-concept study with dupilumab used in CAD is now warranted.
Importantly, there are several case reports of the development of cutaneous T-cell lymphomas and lymphoid infiltrates of unknown significance in atopic patients receiving dupilumab (17), which may indicate accelerated disease progression of cutaneous T-cell lymphoma due to dupilumab, or an unmasking of smouldering cutaneous lymphoma with dupilumab. Hence, we recommend that if mycosis fungoides or Sezary syndrome should be ruled out in general in patients with suspected CAD, this is even more important before dupilumab treatment.
REFERENCES
- Dawe RS, Ferguson J. Diagnosis and treatment of chronic actinic dermatitis. Dermatol Ther 2003; 16: 45-51. https://doi.org/10.1046/j.1529-8019.2003.01607.x
- Burfield L, Rutter KJ, Thompson B, Marjanovic EJ, Neale RE, Rhodes LE. Systematic review of the prevalence and incidence of the photodermatoses with meta-analysis of the prevalence of polymorphic light eruption. J Eur Acad Dermatol Venereol 2023; 37: 511-520. https://doi.org/10.1111/jdv.18772
- Dawe RS, Crombie IK, Ferguson J. The natural history of chronic actinic dermatitis. Arch Dermatol 2000; 136: 1215-1220. https://doi.org/10.1001/archderm.136.10.1215
- Ko DY, Choi SH, Ha SM, Kim TH, Song KH, Hee Kim K, et al, et al. The clinical severity score of chronic actinic dermatitis correlates with in vivo photoallergic reactions and the immunologic parameters related to a shift towards Th2 immunity from the Th2/Th1 balanced status in patients with chronic actinic dermatitis. Photodermatol Photoimmunol Photomed 2016; 32: 199-206. https://doi.org/10.1111/phpp.12244
- Choi KW, Lee CY, Lee YK, Kim YH, Kim KH. A Korean experience with chronic actinic dermatitis during an 18-year period: meteorological and photoimmunological aspects. Photodermatol Photoimmunol Photomed 2009; 25: 286-292 https://doi.org/10.1111/j.1600-0781.2009.00464.x
- Patruno C, Fabbrocini G, Longo G, Argenziano G, Ferrucci SM, Stingeni L, et al. Effectiveness and safety of long-term dupilumab treatment in elderly patients with atopic dermatitis: a multicenter real-life observational study. Am J Clin Dermatol 2021; 22: 581-586. https://doi.org/10.1007/s40257-021-00597-5
- Patel N, Konda S, Lim HW. Dupilumab for the treatment of chronic actinic dermatitis. Photodermatol Photoimmunol Photomed 2020; 36: 398-400. https://doi.org/10.1111/phpp.12566
- Verma L, Pratt M. A case report of therapeutically challenging chronic actinic dermatitis. SAGE Open Med Case Rep 2019; 7: 2050313X19845235. https://doi.org/10.1177/2050313X19845235
- Chen J, Yu N, Wu W, Ou S, Chen Q, Zhu H. The effectiveness and safety of dupilumab for the treatment of recalcitrant chronic actinic dermatitis: a case series. Clin Cosmet Investig Dermatol 2023; 16: 2357-2363. https://doi.org/10.2147/CCID.S422683
- Taboada Paz L, Sanchez Garcia V, Garcia Minarro AM, Ramos Rincon JM, Gil Pallares P, Vicente Basanta E, et al. Chronic actinic dermatitis and fragrance sensitization: case report with good response to dupilumab. Photodermatol Photoimmunol Photomed 2023; 39: 670-672. https://doi.org/10.1111/phpp.12902
- McFeely O, Doyle C, Blasco MC, Beatty P, Murphy L, O’Mahony S, et al. Chronic actinic dermatitis successfully treated with methotrexate and dupilumab. Photodermatol Photoimmunol Photomed 2023; 39: 172-174. https://doi.org/10.1111/phpp.12851
- Ali K, Wu L, Lou H, Zhong J, Qiu Y, Da J, et al. Clearance of chronic actinic dermatitis with dupilumab therapy in Chinese patients: a case series. Front Med 2022; 9: 803692. https://doi.org/10.3389/fmed.2022.803692
- Chen J, Li H, Zhu H. Successful treatment of chronic actinic dermatitis with dupilumab: a case report and review of the literature. Clin Cosmet Investig Dermatol 2021; 14: 1913-1917. https://doi.org/10.2147/CCID.S342401
- Chen JC, Lian CH. Chronic actinic dermatitis in an old adult significantly improved by dupilumab. Photodermatol Photoimmunol Photomed 2022; 38: 176-177. https://doi.org/10.1111/phpp.12731
- Navarro-Triviño FJ, Prados-Carmona A, Aguilera J, de Gálvez MV, Ruiz-Villaverde R. Treatment of refractory solar urticaria: could dupilumab fill the current gap? JDDG J Dtsch Dermatol Ges. 2023; 21: 652-653. https://doi.org/10.1111/ddg.15025
- Perz AM, Fawaz B, Pyle T, Butala N, Green JJ. Dupilumab for the treatment of actinic prurigo. Skinmed 2021; 19: 471-472.
- Hamp A, Hanson J, Schwartz RA, Lambert WC, Alhatem A. Dupilumab-associated mycosis fungoides; a cross-sectional study. Arch Dermatol Res 2023; 315: 2561-2569. https://doi.org/10.1007/s00403-023-02652-z