SHORT COMMUNICATION
Cutaneous T-cell Lymphoma Diagnostic and Therapeutic Trends amidst the COVID-19 Pandemic
Gabriele ROCCUZZO, Nicole MACAGNO, Cristina SARDA, Jelena PISANO, Simone RIBERO, Paolo FAVA# and Pietro QUAGLINO#
Department of Medical Sciences, Section of Dermatology, University of Turin, Via Cherasco 23, IT-10126 Turin, Italy. E-mail: gabriele.roccuzzo@unito.it
#Shared senior authorship.
Citation: Acta Derm Venereol 2024; 104: adv40505. DOI https://doi.org/10.2340/actadv.v104.40505.
Copyright: © 2024 The Author(s). Published by MJS Publishing, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/).
Submitted: Apr 4, 2024; Accepted: Jun 25, 2024; Published: Jul 15, 2024
INTRODUCTION
On 5 May 2023, more than 3 years since its designation as a pandemic, the World Health Organization declared an end to the global public health emergency for COVID-19. Amidst this crisis, healthcare priorities underwent a significant shift towards prioritizing emergency care for acute cases, resulting in the rescheduling or postponement of planned medical treatments, including those cancer-related (1). As for dermato-oncology, the reduction of melanoma diagnoses and related activities during this time has been described, yet reports on the impact on rarer skin cancers such as cutaneous T-cell lymphomas (CTCL) are still scarce (2–4). In the northwestern Italian region of Piedmont, home to approximately 4.3 million residents, the dermatology clinic at the University of Turin has long been the central hub for diagnosing and treating CTCL cases (5, 6). With an incidence rate of 8.66 cases per million individuals annually, our institution typically identifies and manages roughly 35–40 new cases each year. This study seeks to examine the real-life incidence rates, clinical characteristics, and treatment approaches of CTCL patients who sought medical attention during the SARS-CoV-2 pandemic and to investigate any potential differences from the population of CTCL patients analysed just prior to the pandemic outbreak within the same university-based institution.
MATERIALS AND METHODS
The study was conducted in accordance with the Declaration of Helsinki. Patients with the following characteristics were included: first access to our university hospital between 1 January 2020 and 31 December 2022, age > 18, a biopsy-proven diagnosis of CTCL according to the European guidelines, and the presence of complete medical records (7). Stage definition (early: IA–IIA, advanced: IIB–IV), therapy response, and disease progression were evaluated according to standard criteria (8, 9). Patients’ demographics, disease characteristics, and treatments were compared with a reference cohort of CTCL patients conducted right before the pandemic outbreak and previously published (10). This reference group of CTCL patients, referred as the pre-pandemic cohort, underwent treatment at our institution throughout the period from 1 January to 31 December 2019. Mann–Whitney, χ2, and Fisher’s exact tests were used to analyse continuous and paired nominal data, respectively. Survival curves were generated with the Kaplan–Meier method and analysed through a log-rank test. Cox regressions were used to identify factors potentially related to survival in the subgroups of early and advanced stages. The analysis was performed using Stata/SE.v.17 (StataCorp LLC, College Station, TX, USA) (p < 0.05 was considered statistically significant).
RESULTS
During 2020–2022, 72 new cases of CTCL were evaluated. Table I offers a summary of the comparison between patient cohorts from the pre-pandemic and pandemic periods. In terms of demographics, the latter cohort exhibited a higher male prevalence (p < 0.001), while both groups had similar ages at diagnosis (p = 0.060). Notable changes were observed in terms of prolonged median diagnostic delay (32.6 vs 13.5 months, p = 0.043) and shifts in stage at diagnosis, with an increase in the advanced stages (p = 0.013), in both tumoral (p = 0.042) and leukemic forms (Stage IV p = 0.041, B2 p = 0.033). Moreover, a higher incidence of symptomatic cases with pruritus at the time of first evaluation was registered (p < 0.001). As for treatment approaches, compared with the pre-pandemic cohort, a greater proportion of patients received systemic agents such as anti-CD30 brentuximab-vedotin and anti-CCR4 mogamulizumab (15.3% vs 3.2% and 12.5% vs 0.4%, p < 0.001). Conversely, there was a decline in the use of traditional treatments, such as local radiation therapy (p = 0.016), phototherapy (p = 0.001), and peg-interferon-alpha-2a (p = 0.030). The percentage of patients achieving a complete response dropped from 60.1% in the pre-COVID cohort to 41.6% in the 2020–2022 cohort (p = 0.004). During the observation period, none of the included patients presented with SARS-CoV-2-related illnesses. However, the onset of 1 case of primary cutaneous CD4+ small/medium T-cell lymphoproliferative disorder was associated with the administration of the second dose of the BNT162b2 vaccination (16). At data cut-off, 48-month OS rates of 90.1% and 58.2% (p = 0.006) were registered in the early and advanced stage subsets of patients, respectively. On Cox proportional hazards regression, the presence of other non-dermatological cancers (HR 4.47, 95% CI 1.44–13.90, p = 0.010), advanced stage (HR 4.51, 95% CI 1.37–15.11, p = 0.014), and disease progression (HR 6.34, 95% CI 2.00–20.16, p = 0.002) were negatively associated with survival (Fig. 1).
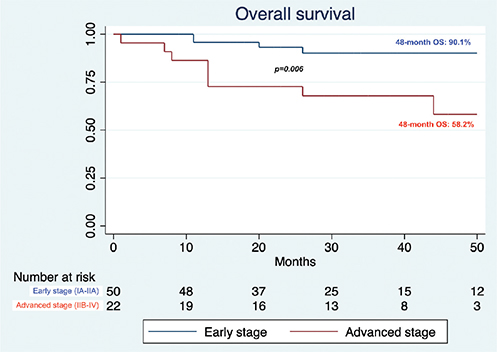
Fig. 1. Overall survival of the 2020–2022 cohort.
DISCUSSION
Over the past 3 years of the COVID-19 pandemic, an extensive body of scientific research has shed light on the intricate relationship between COVID-19 and various dermatological conditions (11). However, the precise impact of this global health crisis on the diagnosis and management of patients with rare skin diseases, such as CTCL, remains uncertain. Within this framework, our study elucidates several notable aspects. First, a notable decline in diagnosed cases was observed, reflecting a decrease of approximately 40% compared with the anticipated 105–120 cases over a 3-year period (5). Conversely, there has been a significant increase in the identification of advanced stages of Mycosis fungoides and Sezary syndrome, alongside a reduction in the attainment of complete responses. This trend might partly result from a reporting bias due to the increase in advanced cases directed to our tertiary-level centre, as oncological access to the facility upon referral from general practitioners remained active throughout the pandemic. Specifically, in the initial year the proportion of advanced cases compared with early cases stood at 60.0%, yet, as social isolation measures were progressively eased, this ratio gradually declined to 33.3%, indicating a gradual restoration of medical access following the first 2 waves, which occurred in February–May and October–December 2020 (2). Second, a notable decline in the utilization of treatment modalities that necessitate multiple hospital visits, such as phototherapy and radiation therapy, has deeply changed the real-life therapeutic scenario. This drop can be attributed both to the pandemic-related restrictions and the patients’ aversion to healthcare facilities, as recently investigated in a Brazilian cross-sectional study in which only 19% of CTCL patients attended phototherapy sessions during periods of social isolation (12). Besides, this shift in treatment patterns may account for the observed increased use of topical steroids as symptom alleviators, in line with the EORTC recommendations released at the end of 2020 (13). Conversely, the availability of interferon was affected by its recent market limitations, leading to supply shortages that likely played a role in the decline in prescriptions, a phenomenon also observed in other settings (14). Concurrently, there has been an upsurge in the adoption of novel systemic agents. While the increased utilization of mogamulizumab could be mainly explained by its timing, as it was predominantly employed in the MAVORIC clinical trial until its reimbursement by the Italian national health system at the end of 2021, the escalated utilization of brentuximab-vedotin is likely secondary to the observed relative rise in CD30+ CTCL cases (9). Notably, pruritus, which may have been more prevalent in the pandemic cohort due to a selection bias favouring patients seeking hospital care for skin discomfort, did not manifest as a negative prognostic factor for survival, unlike our previous report (10). Overall, advanced-stage patients still displayed significantly lower survival rates compared with their early-stage counterparts, highlighting the need for new effective therapeutic agents in the long term (15). As we transition to a post-pandemic world, it is incumbent upon the medical community to thoroughly assess the far-reaching consequences of the pandemic on healthcare delivery
REFERENCES
- De Santis KK, Helmer S, Barnes B, Kraywinkel K, Imhoff M, Müller-Ebersteinet R, et al. Impact of the COVID-19 pandemic on oncological care in Germany: rapid review. J Cancer Res Clin Oncol 2023; 149: 14329-14340. https://doi.org/10.1007/s00432-023-05063-9
- Caini S, Brusasco M, Niero G, De Giorgi V, Lombardo M, Massone C, et al. Healthcare and safety of patients with melanoma during the COVID-19 pandemic in Italy. J Eur Acad Dermatol Venereol 2022; 36: 1669. https://doi.org/10.1111/jdv.18056
- Geskin LJ, Kwinta BD, Garcia-Saleem TJ, Akilov OE, Enz PA, Guenova E, et al. International Society for Cutaneous Lymphomas Pandemic Section (ICLYPS) analysis of cutaneous T-cell lymphoma outcomes during the COVID-19 pandemic: a retrospective cohort study. J Am Acad Dermatol 2023; 88: 935-937. https://doi.org/10.1016/j.jaad.2022.11.016
- Elmasry MF, Youssef R, Elbendary A, Helmy K, Abdelkader HA. Cutaneous lymphomas and COVID-19: what is known so far? Dermatol Ther 2021; 34: e14463. https://doi.org/10.1111/dth.14463
- Willemze R, Cerroni L, Kempf W, Berti E, Facchetti F, Swerdlow SH, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood 2019; 133: 1703-1714. https://doi.org/10.1182/blood-2018-11-881268
- Roccuzzo G, Giordano S, Avallone G, Rubatto M, Canonico S, Funaro A, et al. Sézary syndrome: different erythroderma morphological features with proposal for a clinical score system. Cells 2022; 11: 333. https://doi.org/10.3390/cells11030333
- Olsen EA, Whittaker S, Willemze R, Pinter-Brown L, Francine Foss F, Geskin L, et al. Primary cutaneous lymphoma: recommendations for clinical trial design and staging update from the ISCL, USCLC, and EORTC. Blood 2022; 140: 419-437. https://doi.org/10.1182/blood.2021012057
- Quaglino P, Scarisbrick J, Roccuzzo G, Albeldano A, Battistella M, McCormack C, et al. Identifying unmet needs and challenges in the definition of a plaque in mycosis fungoides: an EORTC-CLTG/ISCL survey. J Eur Acad Dermatol Venereol 2023; 37: 680-688. https://doi.org/10.1111/jdv.18852
- Latzka J, Assaf C, Bagot M, Cozzio A, Dummer R, Guenova E, et al. EORTC consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome: Update 2023. Eur J Cancer 2023; 195: 113343. https://doi.org/10.1016/j.ejca.2023.113343
- Macagno N, Mastorino L, Rubatto M, Avallon G, Merli M, Agostini A, et al. Primary cutaneous lymphoma patients seen at a referral dermatological centre in 1 year: a single-centre observational retrospective cohort study of the diagnoses and staging, comorbidities and associated symptoms, treatment performed and clinical course. J Eur Acad Dermatol Venereol 2022; 36: 2388-2392. https://doi.org/10.1111/jdv.18469
- Avallone G, Quaglino P, Cavallo F, Roccuzzo G, Ribero S, Zalaudek I, et al. SARS-CoV-2 vaccine-related cutaneous manifestations: a systematic review. Int J Dermatol 2022; 61: 1187-1204. https://doi.org/10.1111/ijd.16063
- Costa FB, Baptista PL, Duquia RP. A cross-sectional questionnaire study in a phototherapy unit during COVID-19. Photochem Photobiol Sci 2021; 20: 1239-1242. https://doi.org/10.1007/s43630-021-00093-z
- Papadavid E, Scarisbrick J, Ortiz Romero P, Quaglino P, Vermeer M, Knobler R, et al. Management of primary cutaneous lymphoma patients during COVID-19 pandemic: EORTC CLTF guidelines. J Eur Acad Dermatol Venereol 2020; 34: 1633-1636. https://doi.org/10.1111/jdv.16593
- Jiang J, Böhringer D, Auw-Hädrich C, Maier PC, Barth T, Eter N, et al. Current practice in the treatment of epithelial and melanocytic tumours with interferon-α2b: a survey of tertiary eye centres in Germany. Klin Monbl Augenheilkd 2023; 240: 891-896. https://doi.org/10.1055/a-2029-0163
- Roccuzzo G, Giordano S, Fava P, Pileri A, Guglielmo A, Tonella L, et al. Immune check point inhibitors in primary cutaneous t-cell lymphomas: biologic rationale, clinical results and future perspectives. Front Oncol 2021; 11: 733770. https://doi.org/10.3389/fonc.2021.733770