ORIGINAL REPORT
Identifying Predictors of PASI100 Responses up to Month 12 in Patients with Moderate-to-severe Psoriasis Receiving Biologics in the Psoriasis Study of Health Outcomes (PSoHO)
April W. ARMSTRONG1, Elisabeth RIEDL2, Patrick M. BRUNNER3, Stefano PIASERICO4, Willie I. VISSER5, Natalie HAUSTRUP6, Bruce W. KONICEK6, Zbigniew KADZIOLA6, Mercedes NUNEZ6, Alan BRNABIC6# and Christopher SCHUSTER2,6#
1University of California, Los Angeles, CA, USA, 2Department of Dermatology, Medical University of Vienna, Vienna, Austria, 3Department of Dermatology, Icahn School of Medicine at Mount Sinai, New York, NY, USA, 4Dermatology Unit, Department of Medicine, University of Padova, Padua, Italy, 5Division of Dermatology, Department of Medicine, Tygerberg Academic Hospital and Stellenbosch University, Cape Town, South Africa and 6Eli Lilly and Company, Indianapolis, USA
Despite the abundance of data concerning biologic treatments for patients with psoriasis, clinicians are often challenged with discerning the optimal treatment for each patient. To inform this selection, this study explored whether a patient’s baseline characteristics or disease profile could predict the likelihood of achieving complete skin clearance with biologic treatment. Machine-learning and other statistical methods were applied to the substantial data collected from patients with moderate-to-severe psoriasis in the ongoing, international, prospective, observational Psoriasis Study of Health Outcomes (PSoHO). The 3 measures of complete skin clearance were a psoriasis area and severity index (PASI)100 response at (a) week 12, (b) month 12, and (c) week 12 and maintained at month 6 and month 12 (PASI100 durability). From these real-world data, the absence of nail psoriasis emerged as the most consistent feature that may be used by clinicians to predict high-level treatment responses with biologic treatment. Other significant predictors of skin clearance with biologic treatments were the absence of hypertension and a lower body surface area affected by psoriasis. Overall, this study evidences the substantial challenge of identifying reliable clinical markers of treatment response for patients with psoriasis and highlights the importance of regular screening for psoriatic nail involvement.
Key words: psoriasis; biologics; skin clearance; predictors; PASI100; nail psoriasis.
SIGNIFICANCE
This study explores whether particular features of a patient’s profile could be used to predict the likelihood of achieving complete skin clearance at different timepoints with biologic treatment. The analysis included patients with moderate-to-severe psoriasis enrolled in the large, international, observational Psoriasis Study of Health Outcomes (PSoHO). Although this study identified 26 features, we found that (a) no hypertension, (b) no nail psoriasis, or (c) a lower proportion of the body affected by psoriasis were the most important features in predicting skin clearance with the biologic treatments. In particular, this study highlights the importance of regular screening for psoriatic nail involvement.
Citation: Acta Derm Venereol 2024; 104: adv40556. DOI: https://doi.org/10.2340/actadv.v104.40556.
Copyright: © 2024 The Author(s). Published by MJS Publishing, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/).
Submitted: Apr 25, 2024; Accepted after revision: Aug 12, 2024; Published: Sep 5, 2024
Corr: April Armstrong, University of California, Los Angeles, CA, USA. E-mail: aprilarmstrong@post.harvard.edu
#These authors contributed equally
Competing interests and funding: AWA received grants or contracts from AbbVie, ASLAN, Bristol Myers Squibb, Dermavant Sciences, Dermira, Eli Lilly, Galderma, Incyte, Janssen, LEO Pharma, Meiji, Seika Pharma Co, Modernizing Medicine, Nimbus Therapeutics, Novartis, Ortho Dermatologics, Pfizer, Sanofi Genzyme, UCB, and Ventyx Biosciences; received consulting fees from ASLAN, Almirall, Amgen, Arcutis, Beiersdorf, BMS, Dermavant, EPI Health, Janssen, LEOPharma, Mindera, Nimbus, Organon & Co, Sanofi, Sun Pharma, Takeda, Ventyx Biosciences, and speaking fees from AbbVie, Almirall, Arcutis, ASLAN, Beiersdorf, BI, BMS, EPI, Incyte, LEO, UCB, Janssen, Lilly, Nimbus, Novartis, Ortho Dermatologics, Sun, Dermavant, Dermira, Sanofi, Regeneron, and Pfizer; participated on a data safety monitoring board or advisory board for Boehringer Ingelheim and Parexel; and board of director elect American Academy of Dermatology. ER is a former employee and has pending patents with Eli Lilly and Company and has also been a speaker and/or consultant for Eli Lilly and Company, Pelpharma, Novartis, and Almirall. PMB received consulting fees from LEO Pharma, Pfizer, Sanofi Genzyme, Eli Lilly and Company, Novartis, Celgene, UCB Pharma, Biotest, Boehringer Ingelheim, AbbVie, Amgen, Arena Pharmaceuticals, GSK, and Regeneron; received payment or honoraria for lectures and presentations from LEO Pharma, Pfizer, Sanofi Genzyme, Eli Lilly and Company, Novartis, Celgene, UCB Pharma, Biotest, Boehringer Ingelheim, AbbVie, Amgen, Arena Pharmaceuticals, GSK, and Regeneron; was a board member of the Austrian Society for Allergology and Immunology. SP received consulting fees from AbbVie, Almirall, Celgene, Janssen, LEO Pharma, Eli Lilly and Company, Merck Sharp & Dohme, Novartis, Pfizer, Sandoz, UCB; speaker payments from AbbVie, Almirall, Celgene, Janssen, LEO Pharma, Eli Lilly and Company, Merck Sharp & Dohme, Novartis, Pfizer, Sandoz, and UCB; support for attending meetings or travel from AbbVie, Almirall, Celgene, Janssen, LEO Pharma, Eli Lilly and Company, Merck Sharp & Dohme, Novartis, Pfizer, Sandoz, and UCB. SP received consulting fees from AbbVie, Almirall, Celgene, Janssen, LEO Pharma, Eli Lilly and Company, Merck Sharp & Dohme, Novartis, Pfizer, Sandoz, and UCB; speaker payments from AbbVie, Almirall, Celgene, Janssen, LEO Pharma, Eli Lilly and Company, Merck Sharp & Dohme, Novartis, Pfizer, Sandoz, and UCB; support for attending meetings or travel from AbbVie, Almirall, Celgene, Janssen, LEO Pharma, Eli Lilly and Company, Merck Sharp & Dohme, Novartis, Pfizer, Sandoz, and UCB. WIV received speaking fees from Novartis, Janssen, Eli Lilly and Company, and AbbVie; support for attending meetings from Janssen and Eli Lilly and Company. NH, BK, ZK, MN, AB, and CS are all employees and minor shareholders of Eli Lilly and Company.
This study was funded by Eli Lilly and Company.
INTRODUCTION
The prescription of individual biologics to patients with moderate-to-severe psoriasis (PsO) is still largely based on a trial-and-error approach, despite the abundance of data regarding biologics. Although modern biologics achieve high-level skin responses in a large proportion of patients, this approach can be problematic when the first therapy is not optimal as a patient’s risk of discontinuation due to ineffectiveness increases with repeated biologic switching (1–3). As such, identifying predictors of short- and long-term responsiveness to PsO treatments has significant clinical and economic implications. For example, several studies show that certain demographics (4), comorbidities (5), or involvement of special areas (6) may be associated with a reduction in treatment responses with some medications. By detecting patterns occurring within large quantities of complex patient data, it may be possible to identify, and consequently prescribe, the most appropriate biologic to an individual based on their initial assessment. Building on other studies that accurately predicted the risk of treatment discontinuation based on patient profiles (7–10), this study seeks to identify whether certain baseline demographic and clinical characteristics are associated with increased or decreased odds of achieving complete skin clearance in a real-world setting.
The Psoriasis Study of Health Outcomes (PSoHO) is an ongoing, international, prospective, observational study that was designed to investigate the comparative effectiveness of biologic treatments for patients with moderate-to-severe PsO within a real-world setting (11). Herein, we investigate the baseline profiles of PSoHO patients and their week 12 (11), month 6, and month 12 (12) data with the aim of identifying potential predictors of complete skin clearance at different time points. The 3 measures of complete skin clearance were a psoriasis area and severity index (PASI)100 response at (a) week 12, (b) month 12 and (c) week 12 and maintained at month 6 and month 12 (PASI100 durability). A second objective was to further examine these identified predictor variables to quantify the likelihood of these different PASI100 outcomes and elucidate any differences among biologic classes.
MATERIALS AND METHODS
Psoriasis Study of Health Outcomes (PSoHO)study and patient data
Details of the PSoHO study have been published previously (11, 12). This post-hoc analysis evaluated a sub-population of 1,917 patients with moderate-to-severe PsO who were treated with biologics in 4 treatment classes. These treatment classes were (a) interleukin (IL)-17A (ixekizumab and secukinumab), (b) IL-23 (risankizumab, guselkumab, and tildrakizumab), (c) IL-12/23 (ustekinumab), and (d) tumour necrosis factor (TNF)-α (adalimumab, certolizumab, etanercept, and infliximab) biologics.
Statistical approach to identify candidate predictors of complete skin clearance
First, we evaluated the extensive baseline patient profile data collected in PSoHO to explore potential predictors of complete skin clearance. The 3 measures of complete skin clearance were a PASI100 response at (a) week 12, (b) month 12, and (c) week 12 and maintained at month 6 and month 12, denoted as the PASI100 durability outcome. The study applied a specialized Treatment-Specific Subgroup Detection Tool (TSDT) (13), which is a tree-based tool that identifies subgroups with a superior response relative to the overall sample. In addition, we applied a machine-learning framework with the 3 model strategies of logistic regression (LR), penalized logistic regression (PLR), and extreme gradient boosting (XGBoost). Appendix S1 provides details of these methodologies. In short, the analysis randomly selected 20% of data as the hold-out sample and used the remaining subset to create 1,000 random 70% training and 30% test splits for the evaluation and performance of each model strategy. The performance measures are expressed as median (95% rCI) from 1,000 splits where rCI is the resample confidence interval, i.e., 2.5 and 97.5 percentile. The most important variables were used in the final logistic regression model on the hold-out sample. Hyperparameters for PLR and XGBoost were tuned using 5-fold cross-validation. The analysis used imputation for missing data for potential predictors, and evaluated outcome data as observed (14).
Quantification of the statistical significance of candidate predictors of different PASI100 outcomes
The final analysis quantified the likelihood of skin clearance for each of the predictor variables. A logistic regression model was fitted to the observed PASI100 outcomes using the identified predictors as independent variables. This analysis presents the results as odds ratios (ORs) and 95% confidence intervals (CIs). Outcomes are reported and analysed as observed with no imputation. For further statistical methods, see Appendix S1.
RESULTS
Included patients
Table I provides the baseline demographics, disease characteristics, and treatment history for the overall population of 1,917 patients and for the 4 biologic treatment cohorts. The overall population was 57.7% male, with a mean age of 45.4 years, and a median disease duration of 16.6 years. Of the 1,917 patients, 40.3% received an IL-17A biologic, 34.3% an IL-23 biologic, 18.8% a TNF-α biologic, and 6.6% an IL-12/23 biologic. The profiles of the patients in the IL-23, TNF-α, and IL-12/23 cohorts were largely comparable with those of the IL-17A cohort, with a few exceptions. For example, the IL-17A cohort had a significantly higher number of patients with comorbid psoriatic arthritis (PsA) than the other cohorts. Some other significant differences in baseline patient profiles across cohorts included age, comorbidities, prior biologic use, and concomitant medications.
| Factor | Overall (n=1,917) | IL-17A (n=773) | IL-23 (n=657) | TNF-α (n=360) | IL-12/23 (n=127) |
| Sex, male, n (%) | 1,106 (57.7) | 442 (57.2) | 397 (60.4) | 190 (52.8) | 77 (60.6) |
| Age, years, mean (SD) | 45.4 (13.6) | 46.8 (13.7) | 44.3 (13.5)* | 44.0 (13.2)* | 46.4 (14.5) |
| Weight, kg, mean (SD) | 85.0 (21.1) | 85.6 (20.8) | 84.6 (21.8) | 85.2 (21.5) | 82.9 (17.1) |
| BMI < 30 kg/m2, n (%)a | 1,198 (62.5) | 468 (60.5) | 410 (62.4) | 228 (63.3) | 92 (72.4)** |
| Race, White, n (%) | 1401 (73.1) | 576 (74.5) | 421 (64.1)* | 305 (84.7)* | 99 (78.0) |
| Never smoked, n (%)b | 648 (33.8) | 278 (36.0) | 223 (33.9) | 107 (29.7) | 40 (31.5) |
| Family history of PsO, n (%)c | 780 (40.7) | 302 (39.1) | 283 (43.1) | 148 (41.1) | 47 (37.0) |
| Comorbidities | |||||
| One or more current comorbidities, n (%)d | 1120 (58.4) | 476 (61.6) | 369 (56.2)** | 197 (54.7)** | 78 (61.4) |
| Diabetes mellitus | 210 (11.0) | 93 (12.0) | 72 (11.0) | 29 (8.1) | 16 (12.6) |
| Dyslipidaemia | 338 (17.6) | 145 (18.8) | 119 (18.1) | 56 (15.6) | 18 (14.2) |
| HADs anxiety ≥ 8 | 776 (40.5) | 318 (41.1) | 238 (36.2) | 163 (45.3) | 57 (44.9) |
| HADs depression ≥ 8 | 555 (29.0) | 236 (30.5) | 169 (25.7) | 118 (32.8) | 32 (25.2) |
| Hypertension | 461 (24.1) | 204 (26.4) | 152 (23.1) | 69 (19.2)** | 36 (28.4) |
| Psoriatic arthritise | 445 (23.2) | 227 (29.4) | 121 (18.4)* | 78 (21.7)** | 19 (15.0)* |
| Number of current comorbidities reportedf | 1.5 (1.8) | 1.6 (1.8) | 1.5 (1.8) | 1.3 (1.7)** | 1.7 (2.1) |
| Disease characteristics | |||||
| PASI, mean (SD) | 14.5 (8.6) | 14.6 (8.6) | 14.9 (9.4) | 13.5 (7.0)** | 14.4 (7.9) |
| BSA, % of body surface area | 21.2 (17.7) | 21.1 (17.5) | 21.0 (18.4) | 21.4 (16.9) | 22.6 (17.7) |
| Disease duration, mean (SD) | 16.6 (12.2) | 16.7 (12.4) | 16.8 (11.8) | 16.6 (12.5) | 15.6 (12.1) |
| sPGA, mean (SD) | |||||
| Moderate | 951 (49.6) | 387 (50.1) | 287 (43.7)** | 209 (58.1)** | 68 (53.5) |
| Severe | 592 (30.9) | 242 (31.3) | 221 (33.6) | 92 (25.6) | 37 (29.1) |
| Very severe | 73 (3.8) | 34 (4.4) | 31 (4.7) | 6 (1.7)** | 2 (1.6) |
| DLQI, mean (SD) | 12.6 (7.8) | 12.9 (7.9) | 11.9 (7.7)** | 13.2 (7.6) | 12.3 (8.0) |
| Genital psoriasis, n (%)g | 495 (25.8) | 205 (26.5) | 170 (25.9) | 89 (24.7) | 31 (24.4) |
| Scalp psoriasis, n (%)g | 1,275 (66.5) | 494 (63.9) | 447 (68.0) | 252 (70.0) | 82 (64.6) |
| Palmoplantar psoriasis, n (%)g | 425 (22.2) | 174 (22.5) | 160 (24.4) | 72 (20.0) | 19 (15.0) |
| Nail psoriasis, n (%)g | 731 (38.1) | 305 (39.5) | 253 (38.5) | 128 (35.6) | 45 (35.4) |
| Prior treatment with biologics, n (%)h | 683 (35.6) | 291 (37.7) | 319 (48.6)* | 38 (10.6)* | 35 (27.6)** |
| Concomitant conventional therapy, n (%) | 338 (17.6) | 105 (13.6) | 145 (22.1)* | 66 (18.3)** | 22 (17.3) |
| Concomitant topical therapy, n (%) | 290 (15.1) | 89 (11.5) | 132 (20.1)* | 52 (14.4) | 17 (13.4) |
| PASI100 response rates, % (n/N)i | |||||
| PASI100 at week 12, mean (SD) | 30.4 (516/1,699) | 39.9 (277/694) | 27.7* (164/592) | 18.8* (59/313) | 16.0* (16/100) |
| PASI100 at month 12, mean (SD) | 48.4 (738/1,524) | 50.6 (313/618) | 49.4 (268/543) | 45.4 (122/269) | 37.2** (35/94) |
| PASI100 durability outcome - PASI100 at W12, M6 and M12, mean (SD) | 15.9 (268/1,690) | 20.9 (141/676) | 15.3** (89/580) | 8.4* (27/320) | 9.6** (11/114) |
| Descriptive analyses used Fisher’s exact test or χ2 for categorical variables, and F-test for continuous variables. aMissing data for 31 patients. bMissing data for 268 patients. cMissing data for 50 patients. dMissing data for 4 patients. ePsoriatic arthritis (PsA) diagnosis was recorded by the dermatologists based on the medical history and/or information provided by the patient. fComorbidities were captured based on a pre-defined list. gAssessed by the investigator using a simple yes or no question. h1 patient with missing data in interleukin (IL)-17A group. iPASI100 response rates as observed. | |||||
| BMI: body mass index; BSA: body surface area; PASI: psoriasis area and severity index; Q: quartile; M: month; sPGA: Static Physician Global Assessment; W: week. Where N does not match total patient population, the difference is attributable to missing outcome data. **p-value < 0.05 IL-17A cohort vs other treatment group (shaded in yellow). *p-value < 0.001 IL-17A cohort vs other treatment group (shaded in green). | |||||
Table I also shows the response rates for the three PASI100 outcomes for patients treated with IL-17A, IL-23, IL12/23, or TNF-α biologics, as well as for the overall population. The results showed that a significantly higher proportion of patients in the IL-17A cohort achieved PASI100 at week 12 and the PASI100 durability outcome compared with the 3 other biologic cohorts. For the PASI100 outcome at month 12, the comparison was only significant for IL-17A vs IL-12/23.
Identified predictor variables
This study identified 26 predictor variables for the biologic-treatment population. Machine learning identified 7 variables as predictors of at least 1 of the PASI100 outcomes. These variables included patients who were white, or with no comorbidities, a lower PASI score, or no scalp or palmoplantar PsO.
For completeness, 12 predictor variables were also included based on their clinical relevance according to the literature. Table SI lists the different methods used to identify the final 26 variables that were included in a subsequent logistic regression analysis to quantify the associated likelihood of achieving skin clearance.
In a comparison of the treatment classes, machine learning also found that treatment with IL-17A biologics versus TNF-α or IL-23 were also predictor variables. Table SII provides the hyperparameters of the 3 machine-learning models. Table SIII reports the models’ performance for each PASI100 outcome and shows that, across most measures, all models performed similarly. For example, the area under the receiver operating characteristic (ROC) curve, the area under the curve (AUC) parameter, and the associated 95% CIs were 0.651 (0.604, 0.703) for the XGBoost model, 0.656 (0.609, 0.702) for the LR model, and 0.648 (0.602, 0.696) for the PLR model for the PASI100 at week 12 outcome.
The TSDT analysis identified some of these same predictor variables, as well as contributing a further 7, which were BMI, family history of PsO, PsA, hypertension, nail PsO, and concomitant topical or conventional therapies.
Final logistic regression results
The logistic regression analyses in Fig. 1 show that only 3 out of 26 variables reached statistical significance across the PASI100 outcomes. In the pooled biologic-treated population, patients without hypertension had significantly higher odds of a PASI100 response at week 12. At month 12, the absence of nail PsO and a lower body surface area (BSA) affected by PsO resulted in a higher likelihood of achieving PASI100 response with biologic treatment. The absence of nail PsO was also associated with a higher likelihood of achieving the PASI100 durability outcome.
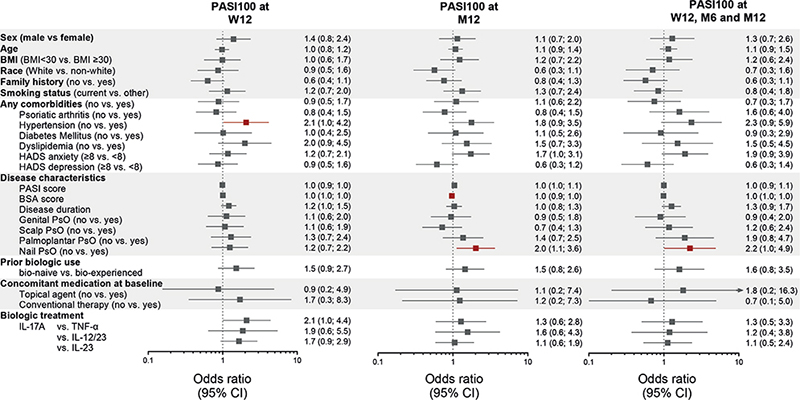
Fig. 1. Odds of achieving 3 PASI100 outcomes for different Psoriasis Study of Health Outcomes (PSoHO) patient demographics, comorbidities, disease characteristics, and treatment variables. Three outcomes were psoriasis area and severity index (PASI)100 at week 12, PASI100 at month 12, or the PASI100 durability outcome. PASI100 durability outcome defined as a PASI100 response at week 12 and maintained at months 6 and 12. Analyses completed using logistic regression. Figure presents data as odds ratios (ORs) and 95% confidence intervals (CIs) and shows outcomes as observed. Red colour indicates when results are statistically significant, whereby the 95% CIs of the ORs do not cross 1. Analysis included 1,917 patients with moderate-to-severe psoriasis (PsO) who were treated with interleukin (IL)-17A, IL-23, IL-12/23, or tumour necrosis factor (TNF)-α biologics. For the hypertension variable for PASI100 at week 12, the lower CI is 1.023. For the body surface area (BSA) variable at month 12, the upper CI is 0.992. For the nail PsO variable for the durability outcome, the lower CI is 1.012. Variables are continuous, unless otherwise stated. Age and disease duration odds are calculated based on 10 units change. Nail, scalp, palmoplantar, and genital psoriasis were evaluated using binary (yes or no) questions. Other smoking status includes patients who never smoked, formally smoked, or had missing data for smoking status. BMI: body mass index; HADS-A: Hospital Anxiety and Depression Scale – Anxiety; HADS-D: Hospital Anxiety and Depression Scale – Depression; M: month; W=week.
DISCUSSION
Building on earlier work that evaluated the comparative effectiveness of different individual biologics included in the PSoHO study (11, 12), this analysis investigated the predictability of complete skin clearance over different time periods using easily accessible clinical information from patients at baseline and included prescribed treatments stratified by class. Using machine-learning and other statistical methods, this study identified 14 predictor variables from the PSoHO sub-population of 1,917 patients with moderate-to-severe PsO treated with biologics, which were added to 12 other variables identified as potential predictors from the literature. A subsequent logistic regression analysis of the 26 variables highlighted 3 key variables that significantly impacted the likelihood of patients treated with biologics achieving at least 1 of the 3 PASI100 outcomes, with nail PsO identified for 2 different outcomes. The other predictor variables were hypertension and BSA.
Psoriatic nail disease is associated with longer PsO duration and greater skin disease severity, and it is a risk factor for the future development of PsA (15). In this study, the absence of nail PsO was the only variable to feature twice, indicating its robustness as a predictor for achieving long-term, durable skin clearance. Moreover, results indicated that biologic-treated patients without nail PsO have approximately 2-times higher odds of achieving PASI100 at month 12 or the durability outcome relative to patients with nail involvement. For patients with nail involvement, it is therefore prudent to consider the performance of different treatments for psoriatic nail disease. It is also worthwhile considering additional assessments to the PASI assessment, which does not evaluate nail PsO (6, 16). Although nail PsO is widely considered to be challenging to treat, biologics have proven efficacious, with IL-17A biologics generally, and ixekizumab specifically, being particularly effective at achieving complete resolution (6, 16, 17). Interestingly, other special areas were not identified as predictors, implying that genital, palmoplantar, and scalp involvement do not substantially impact a patient’s likelihood of achieving PASI100 outcomes in our cohort.
A larger area affected by PsO, as measured by BSA, is a risk factor for the future development of PsA and other chronic comorbidities (18, 19). This study shows that a higher baseline BSA also reduced the likelihood for patients of achieving a PASI100 response at month 12. While BSA serves as a marker for treatment response at month 12, it is noteworthy that it did not predict the other outcomes of interest. Similarly, despite a correlation between BSA and disease severity, as measured by PASI, the baseline PASI score did not significantly affect the likelihood of achieving these outcomes. Future studies will therefore need to untangle the intricate relationship between the various disease severity markers and their potential to serve as biomarkers for clinical response.
PsO and PsA are associated with a greater prevalence of hypertension (20), which, along with other features of the metabolic syndrome, was shown by 1 study to be associated with lower PASI75 and PASI90 responses at 6 months (21). Looking at the PASI100 outcome, we could confirm and extend the association between no hypertension and skin clearance at week 12, although statistical significance was not reached for the other longer-term outcomes. These differences may result from the focus on PASI75 and PASI90 outcomes (21), whereas this analysis applied the more stringent PASI100 measure. Alternatively, the presence of comorbid diseases, such as hypertension, might prompt earlier discontinuation. It is also possible that the impact of elevated pro-inflammatory cytokines, including TNF-a, IL-23, and IL-17A, observed in patients with PsO and hypertension (22) may gradually diminish after successful blockade. As a consequence, hypertension might emerge as a more relevant and pronounced predictor in the short term rather than in the long term.
Of note, many predictors of treatment response previously identified did not emerge as part of this analysis, including various demographic (e.g., sex), disease (e.g., disease duration), and treatment characteristics (e.g., prior biologic use) (4, 5). A possible explanation is the high proportion of modern biologics included in PSoHO that have demonstrated high efficacy for many different patient profiles. Although grouping patients from different treatment classes into a single cohort reduces the granularity of this baseline predictor analysis, the pooling of data allowed for a larger patient population and greater statistical precision. Other limitations of this study include the omission of the IL-17 receptor A treatment class due to low patient numbers, and that comparisons of drug class effectiveness were only completed relative to the IL-17A cohort. Despite the multi-faceted statistical approach to these real-world data, the model performance metrics reflect the difficulty of identifying strong and reliable clinical markers, which aligns with other studies (2, 4). As such, future studies may also consider exploring any association between genetic biomarkers or blood test data with treatment outcomes.
This study evidences the substantial challenge of identifying reliable clinical markers of treatment response for patients with moderate-to-severe PsO. However, the absence of nail PsO emerged as the most consistent feature from these real-world data that may be used by clinicians to easily predict high-level treatment responses with biologic treatment. This principal finding highlights the importance of raising awareness of regular screening for psoriatic nail involvement and to consider the most appropriate treatment for the patient based on this assessment.
ACKNOWLEDGEMENTS
The authors thank the participants, caregivers, and investigators of this study.
Ethics approval: The protocol, amendments, and consent documentation were approved by local institutional review boards (IRB). The study was registered at the European Network of Centers for Pharmacoepidemiology and Pharmacovigilance (ENCEPP24207) and was conducted according to the International Conference on Harmonization, Good Clinical Practice guidelines, and the Declaration of Helsinki. All patients were required to give informed consent for participation in the study. We confirm that the necessary central or local IRB and/or ethics committee approvals have been obtained for this multi-site, international study by United BioSource LLC (UBC). Approvals can be provided on request.
Data availability statement: Data are available on reasonable request. Lilly provides access to all individual participant data collected during the study, after anonymization. Data are available to request after primary publication acceptance. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
REFERENCES
- Egeberg A, Ottosen M, Gniadecki R, Broesby-Olsen S, Dam T, Bryld LE, et al. Safety, efficacy and drug survival of biologics and biosimilars for moderate-to-severe plaque psoriasis. Br J Dermatol 2018; 178: 509–519. https://doi.org/10.1111/bjd.16102
- Gniadecki R, Bang B, Bryld L, Iversen L, Lasthein S, Skov L. Comparison of long-term drug survival and safety of biologic agents in patients with psoriasis vulgaris. Br J Dermatol 2015; 172: 244–252. https://doi.org/10.1111/bjd.13343
- Mason KJ, Barker JN, Smith CH, Hampton PJ, Lunt M, McElhone K, et al. Comparison of drug discontinuation, effectiveness, and safety between clinical trial eligible and ineligible patients in BADBIR. JAMA Dermatol 2018; 154: 581–588. https://doi.org/10.1001/jamadermatol.2018.0183
- Warren RB, Marsden A, Tomenson B, Mason KJ, Soliman MM, Burden AD, et al. Identifying demographic, social and clinical predictors of biologic therapy effectiveness in psoriasis: a multicentre longitudinal cohort study. Br J Dermatol 2019; 180: 1069–1076. https://doi.org/10.1111/bjd.16776
- Lynde C, Riedl E, Maul J-T, Torres T, Pinter A, Fabbrocini G, et al. Comparative effectiveness of biologics across subgroups of patients with moderate-to-severe plaque psoriasis: results at week 12 from the PSoHO Study in a real-world setting. Adv Ther 2023; 40: 869–886. https://doi.org/10.1007/s12325-022-02379-9
- Piaserico S, Riedl E, Pavlovsky L, Vender RB, Mert C, Tangsirisap N, et al. Comparative effectiveness of biologics for patients with moderate-to-severe psoriasis and special area involvement: week 12 results from the observational Psoriasis Study of Health Outcomes (PSoHO). Front Med 2023; 10: 1185523. https://doi.org/10.3389/fmed.2023.1185523
- Du AX, Ali Z, Ajgeiy KK, Dalager MG, Dam TN, Egeberg A, et al. Machine learning model for predicting outcomes of biologic therapy in psoriasis. J Am Acad Dermatol 2023; 88: 1364–1367. https://doi.org/10.1016/j.jaad.2022.12.046
- Emam S, Du AX, Surmanowicz P, Thomsen SF, Greiner R, Gniadecki R. Predicting the long-term outcomes of biologics in patients with psoriasis using machine learning. Br J Dermatol 2020; 182: 1305–1307. https://doi.org/10.1111/bjd.18741
- Nielsen ML, Petersen TC, Maul JT, Wu JJ, Rasmussen MK, Bertelsen T, et al. Multivariable predictive models to identify the optimal biologic therapy for treatment of patients with psoriasis at the individual level. JAMA Dermatol 2022; 158: 1149–1156. https://doi.org/10.1001/jamadermatol.2022.3171
- Brnabic A, Hess LM. Systematic literature review of machine learning methods used in the analysis of real-world data for patient-provider decision making. BMC Med Inform Decis Mak 2021; 21: 54. https://doi.org/10.1186/s12911-021-01403-2
- Pinter A, Puig L, Schäkel K, Reich A, Zaheri S, Costanzo A, et al. Comparative effectiveness of biologics in clinical practice: week 12 primary outcomes from an International Observational Psoriasis Study of Health Outcomes (PSoHO). J Eur Acad Dermatol Venereol 2022; 36: 2087–2100. https://doi.org/10.1111/jdv.18376
- Pinter A, Costanzo A, Khattri S, Smith SD, Carrascosa JM, Tada Y, et al. Comparative effectiveness and durability of biologics in clinical practice: month 12 outcomes from the International, Observational Psoriasis Study of Health Outcomes (PSoHO). Derm Ther 2024; 14: 1479–1493. https://doi.org/10.1007/s13555-023-01086-9
- Shen L, Ding Y, Battioui C. A framework of statistical methods for identification of subgroups with differential treatment effects in randomized trials. Applied Statistics in Biomedicine and Clinical Trials Design: Selected Papers from 2013 ICSA/ISBS Joint Statistical Meetings. Dordrecht: Springer, 2015: p. 411–425. https://doi.org/10.1007/978-3-319-12694-4_25
- Tang F, Ishwaran H. Random forest missing data algorithms. Statistical Analysis and Data Mining: The ASA Data Science J 2017; 10: 363–377. https://doi.org/10.1002/sam.11348
- Canal-García E, Bosch-Amate X, Belinchón I, Puig L. Nail psoriasis. Actas Dermosifiliogr 2022; 113: T481–T490. https://doi.org/10.1016/j.ad.2022.01.032
- Egeberg A, Kristensen LE, Puig L, Rich P, Smith SD, Garrelts A, et al. Network meta-analyses comparing the efficacy of biologic treatments for achieving complete resolution of nail psoriasis at 24–28 and 48–52 weeks. J Derm Treatment 2023; 34: 2263108. https://doi.org/10.1080/09546634.2023.2263108
- Riedl E, Pinter A, Zaheri S, Costanzo A, Brnabic A, Konicek B, et al. Baseline characteristics and mNAPSI change from baseline scores through month 12 for patients with moderate-to-severe plaque psoriasis and concomitant nail psoriasis treated with biologics from PSoHO. Dermatol Ther 2024; 14: 1327–1335. https://doi.org/10.1007/s13555-024-01150-y
- Ogdie A, Shin DB, Love TJ, Gelfand JM. Body surface area affected by psoriasis and the risk for psoriatic arthritis: a prospective population-based cohort study. Rheumatology 2022; 61: 1877–1884. https://doi.org/10.1093/rheumatology/keab622
- Wilson FC, Icen M, Crowson CS, McEvoy MT, Gabriel SE, Kremers HM. Incidence and clinical predictors of psoriatic arthritis in patients with psoriasis: a population-based study. Arthritis Rheum 2009; 61: 233–239. https://doi.org/10.1002/art.24172
- Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and hypertension: a systematic review and meta-analysis of observational studies. J Hypertens 2013; 31: 433–442; discussion 442–443. https://doi.org/10.1097/HJH.0b013e32835bcce1
- Enos CW, Ramos VL, McLean RR, Lin TC, Foster N, Dube B, et al. Comorbid obesity and history of diabetes are independently associated with poorer treatment response to biologics at 6 months: a prospective analysis in Corrona Psoriasis Registry. J Am Acad Dermatol 2022; 86: 68–76. https://doi.org/10.1016/j.jaad.2021.06.883
- Hu MY, Yang Q, Zheng J. The association of psoriasis and hypertension: focusing on anti-inflammatory therapies and immunological mechanisms. Clin Exp Dermatol 2020; 45: 836–840. https://doi.org/10.1111/ced.14327