ORIGINAL REPORT
Nevus Spilus, Partial Unilateral Lentiginosis, and Linear and Whorled Nevoid Hypermelanosis: A Comparison of Clinical Features, Course, and Treatment Response
Hui-Ting HAN1, Jung-Je PARK1, Ji Su LEE2 and Si-Hyung LEE1–3
1Department of Dermatology, Seoul National University College of Medicine, Seoul, 2Department of Dermatology, Seoul National University Hospital, Seoul, and 3Institute of Human-Environmental Interface Biology, Medical Research Center, Seoul National University, Seoul, Korea
Skin diseases manifesting as agminated pigmented lesions have overlapping clinical manifestations. Therefore, accurate differentiation is challenging. The clinical characteristics, histopathological findings, and treatment response of patients diagnosed with partial unilateral lentiginosis, nevus spilus, or linear and whorled nevoid hypermelanosis were retrospectively analysed. Each disease demonstrated distinct demographic and clinical characteristics, and the responses to laser treatment varied. The median age at onset varied significantly among the groups: 0.1, 6.6, and 0.5 years in patients with nevus spilus, partial unilateral lentiginosis, and linear and whorled nevoid hypermelanosis, respectively. Regarding the locations of the skin lesions, partial unilateral lentiginosis occurred predominantly on the head and neck, while approximately half of nevus spilus and linear and whorled nevoid hypermelanosis were observed on the extremities. Although linear and whorled nevoid hypermelanosis and partial unilateral lentiginosis share a similar histological feature of basal hyperpigmentation, patients with linear and whorled nevoid hypermelanosis showed the best response to laser treatment, while patients with partial unilateral lentiginosis demonstrated a poor treatment response. The study’s data may provide important clues for the differential diagnosis and clinical decision-making regarding the treatment of these agminated pigmented lesions.
SIGNIFICANCE
Differentiating disorders with agminated pigment lesions, such as nevus spilus, partial unilateral lentiginosis, and linear and whorled nevoid hypermelanosis, is usually straightforward when they exhibit typical clinical presentations. However, the clinical manifestations are diverse, leading to similar appearances and challenging differential diagnoses. This study provides clues for the differential diagnosis and compares treatment responses among these 3 conditions.
Key words: diagnosis; differential; laser therapy; pigmentation; treatment outcome.
Citation: Acta Derm Venereol 2024; 104: adv40565. DOI https://doi.org/10.2340/actadv.v104.40565.
Copyright: © 2024 The Author(s). Published by MJS Publishing, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/).
Submitted: Apr 15, 2024; Accepted after revision: Jul 3, 2024; Published: Aug 23, 2024
Corr: Si-Hyung Lee, MD, PhD, Department of Dermatology, Seoul National University Hospital, 101, Daehak-ro, Jongno-gu, Seoul 03080, Korea. Email: shleedm@snu.ac.kr
Competing interests and funding: The authors have no conflicts of interest to declare.
INTRODUCTION
Pigmented macules or papules sometimes appear in clusters, even with segmental distributions. In 1904, McKelway described grouped pigmentary lesions arranged in a segmental pattern, which were subsequently named partial unilateral lentiginosis (PUL) (1). PUL is a rare cutaneous pigmentary disorder characterized by agminated lentigines and is mostly found with a unilateral segmental distribution (1, 2). In addition to PUL, there are several skin disorders with multiple pigmented macules and/or papules in an agminated pattern, including nevus spilus (NS) (3, 4) and linear and whorled nevoid hypermelanosis (LWNH) (5, 6). Because of their similar clinical appearances, they are easily confused, sometimes making accurate diagnosis difficult. NS is characterized by a brown hyperpigmented macule with overlapping, darker speckles and papules (7). NS is histologically distinguished from PUL by the presence of dense spots showing histological features of junctional or compound nevus, while the dense spots of PUL usually exhibit lentiginous hyperplasia (8). However, when the colour of the darker spots within an NS lesion is not significantly darker or flat, distinguishing it from PUL is often challenging. Furthermore, if a large NS lesion exhibits a flag, block, or checkerboard pattern, differential diagnosis from PUL is challenging (9, 10). LWNH is a rare pigmentary disorder characterized by hyperpigmented macules and patches with a linear or whorled pattern, often following the lines of Blaschko (6, 11). In addition, PUL lesions can also appear linearly along the lines of Blaschko, and especially when the brown background patch of the PUL is faint or not clearly visible, and the lentigines are densely positioned, it becomes difficult to distinguish them from LWNH.
Distinguishing between these rare diseases is difficult because of the similarities in their clinical manifestations. Accordingly, we aimed to investigate the clinical characteristics of these 3 entities and identify differentiating factors that can aid in the diagnostic process. In addition, we aimed to evaluate the treatment response of each disease to facilitate the development of an appropriate treatment plan for each condition.
MATERIALS AND METHODS
Patients
Among 172 patients who visited the Department of Dermatology of Seoul National University Hospital (Seoul, Korea) between 2000 and 2021 with agminated pigmentary lesions, we enrolled 105 patients who showed a typical clinical presentation of PUL, LWNH, or NS based on clinicopathological evaluation. These diagnoses were made blindly by 2 experienced, board-certified dermatologists (SHL and JSL) who reviewed the patients’ clinical photographs. Among 172 patients, 16 were excluded due to the absence of photographs, and 51 were excluded due to their atypical clinical presentation or failure of the 2 experts to reach a consensus. This study was approved by the Institutional Review Board of the Seoul National University Hospital (No. 2107-059-1233).
Clinical information
Clinical data on sex, age at onset, congenital or acquired nature, lesion progression, symptoms, distribution, and response to laser treatment were assessed by reviewing each patient’s medical records and clinical photographs.
Methods of laser treatment and treatment response
We investigated the types of lasers used on patients undergoing laser treatment and evaluated the treatment response for dermatologists using the following methods. The 5-point scale (1–5) was as follows: 1, no improvement (0%); 2, improvement of 1–25%; 3, improvement of 26–50%; 4, improvement of 51–75%; and 5, improvement of 76–100%. A total of 34 patients were treated with laser therapy, including 9 with NS, 20 with PUL, and 5 with LWNH. The average number of laser treatments performed was 4; patients with NS received 5 treatments and patients with PUL and those with LWNH received 3 treatments. The average treatment duration for all patients was 186 days; the average treatment duration was 261, 153, and 190 days for patients with NS, PUL, and LWNH, respectively. Only patients whose treatment response could be assessed using photographs were included in the treatment response analysis.
Histological features
Histopathological assessment was conducted on skin biopsies collected from 30 patients, including 9 cases of NS, 18 cases of PUL, and 3 cases of LWNH. Nest nevus cells, melanocyte hyperplasia, basal melanin hyperpigmentation, dermal melanophages, elongated rete ridges, and superficial perivascular inflammatory cells were assessed.
Statistical analysis
The collected data were analysed using R software version 4.3.1 (R Foundation for Statistical Computing, Vienna, Austria). The results were analysed using descriptive statistics, Fisher’s exact test, the Kruskal–Wallis test, and the Dunn–Bonferroni post hoc test for differences and significance between the groups. P < 0.05 indicated a statistically significant difference.
RESULTS
Clinical and demographic features
Among the 105 patients, 25 were diagnosed with NS, characterized by multiple dark-brown to black macules or papules appearing on café-au-lait-like flat patches. Further, 58 patients were diagnosed with PUL, characterized by multiple light-to dark-brown macules on normal skin or with a light-brown background. Moreover, 22 patients were diagnosed with LWNH, characterized by hyperpigmented macules or patches arranged in a linear or whorled streaky configuration. All patients showed typical clinical presentation, and the diagnosis of each condition was straightforward. The demographic and clinical features of the patients are summarized in Table I. Most patients were female, although a significantly lower proportion of females was found in the NS group relative to the PUL group (p = 0.038) and the LWNH group (p = 0.022). The median age of onset was significantly lower in the NS group (0.1 years) than in the PUL group (6.6 years) (p = 0.006). Lesions were congenital in 11 (78.6%), 9 (23.1%), and 6 (33.3%) patients in the NS, PUL, and LWNH groups, respectively (between-group comparison: p = 0.001). Six (60.0%), 21 (80.8%), and 7 (100%) patients in the NS, PUL, and LWNH groups, respectively, showed lesion progression following the initial presentation. Only 1 patient each in the NS and LWNH groups complained of pain, whereas the majority of the patients were asymptomatic.
| Factor | NS n = 25 | PUL n = 58 | LWNH n = 22 | Overall p-value |
| Sex, n (%) | 0.018* | |||
| Female | 10 (40.0) | 39 (67.2) | 17 (77.3) | |
| Male | 15 (60.0) | 19 (32.8) | 5 (22.7) | |
| Age at onset, years, median [Q1, Q3] | 0.1 [0.03;0.43] | 6.6 [0.07;13.6] | 0.5 [0.13;1.16] | 0.007* |
| Congenital, n (%) | 11 (78.6) | 9 (23.1) | 6 (33.3) | 0.001* |
| Disease progression, n (%) | 6 (60.0) | 21 (80.8) | 7 (100) | 0.178 |
| Symptoms, n (%) | 0.206 | |||
| No symptoms | 10 (90.9) | 21 (100) | 6 (85.7) | |
| Pain | 1 (9.09) | 0 (0.0) | 1 (14.3) | |
| Overall p-values by the Kruskal–Wallis test for continuous variables and Fisher’s test for categorical variables. | ||||
| NS: nevus spilus; PUL: partial unilateral lentiginosis; LWNH: linear and whorled nevoid hypermelanosis. *p < 0.05. | ||||
Location of the lesions
The location of the lesions varied (Table II) and differed significantly among the 3 groups (p < 0.001). The anatomical sites of each lesion differed depending on the diagnosis. When the anatomical sites were categorized into head and neck, trunk, and extremities, PUL lesions were most commonly observed on the head and neck (51.0%), while NS and LWNH lesions were most commonly observed on the extremities (46.4% and 50.0%, respectively).
| Location of lesion | NS N = 28 n (%) | PUL N = 102 n (%) | LWNH N = 38 n (%) | Overall p-value |
| Head and neck | 7 (25.0) | 52 (51.0) | 13 (34.2) | < 0.001* |
| Face | 5 (17.9) | 33 (32.4) | 11 (28.9) | |
| Neck | 2 (7.1) | 19 (18.6) | 2 (5.3) | |
| Trunk | 8 (28.6) | 35 (34.3) | 6 (15.8) | |
| Chest | 0 (0.0) | 7 (6.9) | 1 (2.6) | |
| Abdomen | 2 (7.1) | 10 (9.8) | 2 (5.3) | |
| Back | 4 (14.3) | 11 (10.8) | 3 (7.9) | |
| Axilla | 0 (0.0) | 5 (4.9) | 0 (0.0) | |
| Inguinal | 2 (7.1) | 2 (2.0) | 0 (0.0) | |
| Extremities | 13 (46.4) | 15 (14.7) | 19 (50.0) | |
| Arm | 3 (10.7) | 6 (5.9) | 8 (21.1) | |
| Hand | 0 (0.0) | 2 (2.0) | 2 (5.3) | |
| Buttock | 2 (7.1) | 1 (1.0) | 1 (2.6) | |
| Leg | 6 (21.4) | 4 (3.9) | 7 (18.4) | |
| Foot | 2 (7.1) | 2 (2.0) | 1 (2.6) | |
| If a patient had lesions at more than 2 anatomical sites, each lesion was counted separately. | ||||
| NS: nevus spilus; PUL: partial unilateral lentiginosis; LWNH: linear and whorled nevoid hypermelanosis. *p < 0.05. | ||||
Histopathological features
Of the patients who underwent skin biopsy for diagnostic purposes, we analysed histopathological findings in 30 (Table III). As expected, nevus cell nests were observed only in the NS group, while melanocyte hyperplasia (9.1%), basal hyperpigmentation (18.2%), and elongated rete ridges (9.1%) were also observed in the NS group. The PUL group showed melanocyte hyperplasia (2.9%), basal hyperpigmentation (50.0%), dermal melanophages (26.5%), perivascular lymphocyte infiltration (17.6%), and elongated rete ridges (2.9%). All lesions in the LWNH group exhibited basal hyperpigmentation. The histology of lesions differed significantly among the 3 groups (p < 0.001).
| Factor | NS n = 11 | PUL n = 34 | LWNH n = 3 | Overall p-value |
| < 0.001* | ||||
| Nests of nevus cells | 7 (63.6) | 0 (0.0) | 0 (0.0) | |
| Melanocytes hyperplasia | 1 (9.1) | 1 (2.9) | 0 (0.0) | |
| Basal hyperpigmentation | 2 (18.2) | 17 (50.0) | 3 (100) | |
| Dermal melanophages | 0 (0.0) | 9 (26.5) | 0 (0.0) | |
| Perivascular lymphocytes infiltration | 0 (0.0) | 6 (17.6) | 0 (0.0) | |
| Elongated rete ridges | 1 (9.1) | 1 (2.9) | 0 (0.0) | |
| NS: nevus spilus; PUL: partial unilateral lentiginosis; LWNH: linear and whorled nevoid hypermelanosis. *p < 0.05. | ||||
Laser treatment response
The details of the laser treatment and treatment responses are summarized in Table IV. For the treatment of these patients, a Q-switched Nd:YAG laser, picosecond Nd:YAG laser, CO2 laser, and intense pulsed light were primarily used, and there was no difference in the types and frequencies of the lasers used among the groups. An improvement of > 50% was experienced by 5 (55.5%) patients in the NS group and none in the PUL group. Although the number of patients was small, all patients in the LWNH group showed an improvement of > 75%. A significant difference in the treatment response was observed between groups (p < 0.001), with pairwise comparisons revealing a significant difference between the NS and PUL groups (p < 0.001) and between the PUL and LWNH groups (p < 0.001).
| Factor | NS n = 10 | PUL n = 27 | LWNH n = 6 | Overall p-value |
| Laser types | 0.196 | |||
| IPL | 1 (10.0) | 1 (3.7) | 1 (16.7) | |
| Pico Nd:YAG | 0 (0.0) | 6 (22.2) | 0 (0.0) | |
| Q-switched Nd:YAG | 7 (70.0) | 19 (70.4) | 5 (83.3) | |
| CO2 laser | 2 (20.0) | 1 (3.7) | 0 (0.0) | |
| Treatment response | <0.001* | |||
| 1 (0%) | 0 (0.0) | 17 (65.4) | 0 (0.0) | |
| 2 (1–25%) | 2 (22.2) | 8 (30.8) | 0 (0.0) | |
| 3 (26–50%) | 2 (22.2) | 1 (3.8) | 0 (0.0) | |
| 4 (51–75%) | 3 (33.3) | 0 (0.0) | 0 (0.0) | |
| 5 (76–100%) | 2 (22.2) | 0 (0.0) | 3 (100) | |
| Some patients received multiple treatments, which were counted separately. | ||||
| Only patients whose treatment responses could be assessed using photographs were included. Fisher’s exact test was used to analyse categorical variables. | ||||
| NS: nevus spilus; PUL: partial unilateral lentiginosis; LWNH: linear and whorled nevoid hypermelanosis. *p < 0.05. | ||||
DISCUSSION
In this study, by comparing the clinical presentations of NS, PUL, and LWNH (Fig. 1A–C), we provide clues for the differential diagnosis and report the response to laser treatment of each disorder. A comparable number of males and females comprised the NS group, whereas a higher proportion of females was observed in the PUL and LWNH groups. This finding is consistent with that of previous studies, which have shown that, regardless of race, NS is observed in similar proportions among males and females; meanwhile, PUL has a higher proportion of female patients (2, 8, 12). This may indeed reflect a higher incidence among females with PUL. However, there could also be bias, considering that most studies have been conducted on patients visiting hospitals. As approximately 78.6% of the NS cases in our study were congenital, they were more likely to be recognized by caregivers at birth or in early infancy, leading to a higher likelihood of hospital visits. In contrast, PUL mostly occurs later in childhood; therefore, women, who may be more concerned about appearance-related issues, might visit hospitals more frequently. Further studies are required to explore these aspects.
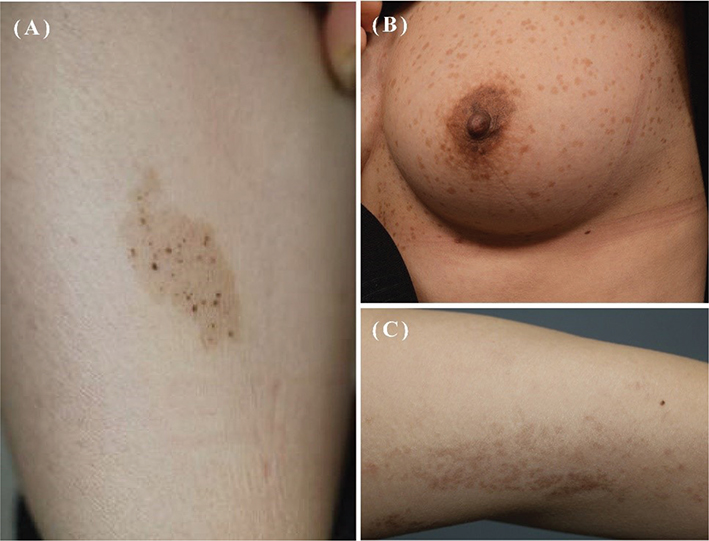
Fig. 1. (A) A typical image of nevus spilus characterized by dark macules and papules within a brown patch resembling café au lait macule. (B) A typical image of partial unilateral lentiginosis showing multiple lentigines within a light brown patch. (C) A typical image of linear and whorled nevoid hypermelanosis presenting brown-coloured macules and patches arranged in a linear or whorled streaky pattern.
In this study, the median age of onset in the NS group was 0.1 years, and 11 (78.6%) cases were congenital. According to the literature, NS is commonly found at birth or within the first month; therefore, NS can be classified as a congenital nevus (7, 13). As NS may initially appear as subtle tan spots at birth or in early infancy, progressing to more noticeable pigmented black, brown, or red-brown macules and papules over months or years (14), some NS lesions may be found late and could thus be classified as acquired, despite being present since birth. In our study, 21.4% were classified as non-congenital and, among these, some may have been congenital but discovered late.
Similarly, the median age of LWNH onset was 0.5 years in our study. This finding is consistent with that of a previous report showing LWNH manifests within the first few months of life, progresses gradually during the first 1–2 years, and then enters the stabilization phase (6). In contrast, PUL in our current study had a median onset age of 6.6 years, consistent with previous research that reported a median age of onset of 5 years for PUL (8, 15). Overall, PUL tends to occur around the age of 5–6 years on average, whereas NS and LWNH are often detected at birth or within the first few months of life. This distinction could be important for the differential diagnosis.
Regarding the locations of the skin lesions, PUL occurred predominantly on the head and neck, while approximately half of NS and LWNH were observed on the extremities. Additionally, LWNH was relatively rarely observed on the trunk, and PUL was infrequently observed on the extremities. These findings are consistent with those of previous studies, which showed that PUL lesions can occur at various body sites, including the face, neck, trunk, and limbs; however, the neck is specifically noted as the most common location of PUL lesions (2, 16). NS lesions are typically located on the trunk and extremities, especially the upper limbs (14). These anatomical differences may be important considerations for distinguishing the 3 conditions.
Although the number of cases in this study was limited, the LWNH group demonstrated an excellent response to laser therapy. The NS group showed > 50% improvement in over half of the cases. However, among the 27 cases of PUL, no patient showed an improvement of > 50%. While LWNH and PUL share a similar histological feature of basal hyperpigmentation, as noted in our study, the response to laser treatment was significantly different. This finding suggests that differences in pathogenesis may be the underlying cause, and further studies are required.
Our study had some limitations. First, this was a retrospective study conducted at a single tertiary centre. Second, only Korean patients were included, which limited the generalizability of the findings. Third, the sample size was relatively small, and further large-scale studies are required to confirm our results. Despite these limitations, to the best of our knowledge the present study is the largest-scale analysis comparing 3 skin disorders (NS, PUL, and LWNH) manifesting as agminated pigmented lesions, which may appear clinically similar and pose challenges in differentiation.
In summary, our study found distinctive clinical characteristics, such as the age of onset, location of the lesion, and laser treatment response, for each of these 3 diseases. These distinctions provide important clues for the differential diagnosis of these diseases. Patients with LWNH exhibit an excellent response to laser therapy, indicating that laser therapy is the treatment of choice. In contrast, PUL exhibits a very poor response to laser treatment, which should be considered crucial in treatment decision-making. This finding is important to prevent the waste of time and resources due to ineffective treatment.
ACKNOWLEDGEMENTS
Reviewed and approved by the Institutional Review Board of Seoul National University Hospital (No.2107-059-1233).
REFERENCES
- Trattner A, Metzker A. Partial unilateral lentiginosis. J Am Acad Dermatol 1993; 29: 693–695. https://doi.org/10.1016/0190-9622(93)70232-I
- Yaşar Ş, Ersanli A, Göktay F, Aytekin S, Cebeci D, Güneş P. Partial unilateral lentiginosis is mosaic neurofibromatosis type 1 or not? J Dermatol 2017; 44: 29–35. https://doi.org/10.1111/1346-8138.13510
- Happle R. Speckled lentiginous naevus: which of the two disorders do you mean? Clin Exp Dermatol 2009; 34: 133–135. https://doi.org/10.1111/j.1365-2230.2008.02966.x
- Prodinger C, Tatarski R, Laimer M, Ahlgrimm-Siess V. Large congenital nevus spilus: improved follow-up through the use of in vivo reflectance confocal microscopy. Dermatol Pract Concept 2013; 3: 55. https://doi.org/10.5826/dpc.0302a08
- Catherine S, Lacour J-P, Passeron T. Treatment of linear and whorled hypermelanosis with Q-switched laser. Dermatol Surg 2014; 40: 1044–1046. https://doi.org/10.1097/01.DSS.0000452635.06834.f5
- Kalter DC, Griffiths WA, Atherton DJ. Linear and whorled nevoid hypermelanosis. J Am Acad Dermatol 1988; 19: 1037–1044. https://doi.org/10.1016/S0190-9622(88)70269-8
- Torres KG, Carle L, Royer M. Nevus spilus (speckled lentiginous nevus) in the oral cavity: report of a case and review of the literature. Am J Dermatopathol 2017; 39: e8–e12. https://doi.org/10.1097/DAD.0000000000000647
- Kim HT, Choi ME, Na H, Lee WJ, Won CH, Lee MW, et al. Partial unilateral lentiginosis: a clinicopathological analysis of 32 cases on the head and neck area in Korea. Int J Dermatol 2021; 60: 1376–1384. https://doi.org/10.1111/ijd.15630
- Barysch MJ, Dummer R. The spectrum of widespread hyperpigmentations from SLN to SUL. Arch Dermatol 2009; 145: 953–954. https://doi.org/10.1001/archdermatol.2009.163
- Happle R. Speckled lentiginous nevi: no longer one single disorder. Arch Dermatol 2010; 146: 204–204. https://doi.org/10.1001/archdermatol.2009.393
- Fernandes C, Waddell A, Jean S-É. Linear and whorled nevoid hypermelanosis and Joubert syndrome: a novel association: A case report and literature review. SAGE Open Med Case Rep 2019; 7: 2050313X19876725. https://doi.org/10.1177/2050313X19876725
- Brito MHTSd, Dionísio CSNdM, Fernandes CMBM, Ferreira JCM, Rosa MJMdPMd, Garcia MMAPdS. Synchronous melanomas arising within nevus spilus. An Bras Dermatol 2017; 92: 107–109. https://doi.org/10.1590/abd1806-4841.20175230
- Cohen LM. Nevus spilus: congenital or acquired? Arch Dermatol 2001; 137: 215–216.
- Vaidya DC, Schwartz RA, Janniger CK. Nevus spilus. Cutis 2007; 80: 465.
- Kim EH, Kang HY. Partial unilateral lentiginosis with ocular involvement. Eur J Dermatol 2006; 16: 582–583.
- Park YM, Kang H, Cho SH, Cho BK. Partial unilateral lentiginosis: clinicopathologic review of 13 cases. Ann Dermatol 2000; 12: 90–94. https://doi.org/10.5021/ad.2000.12.2.90